Abstract
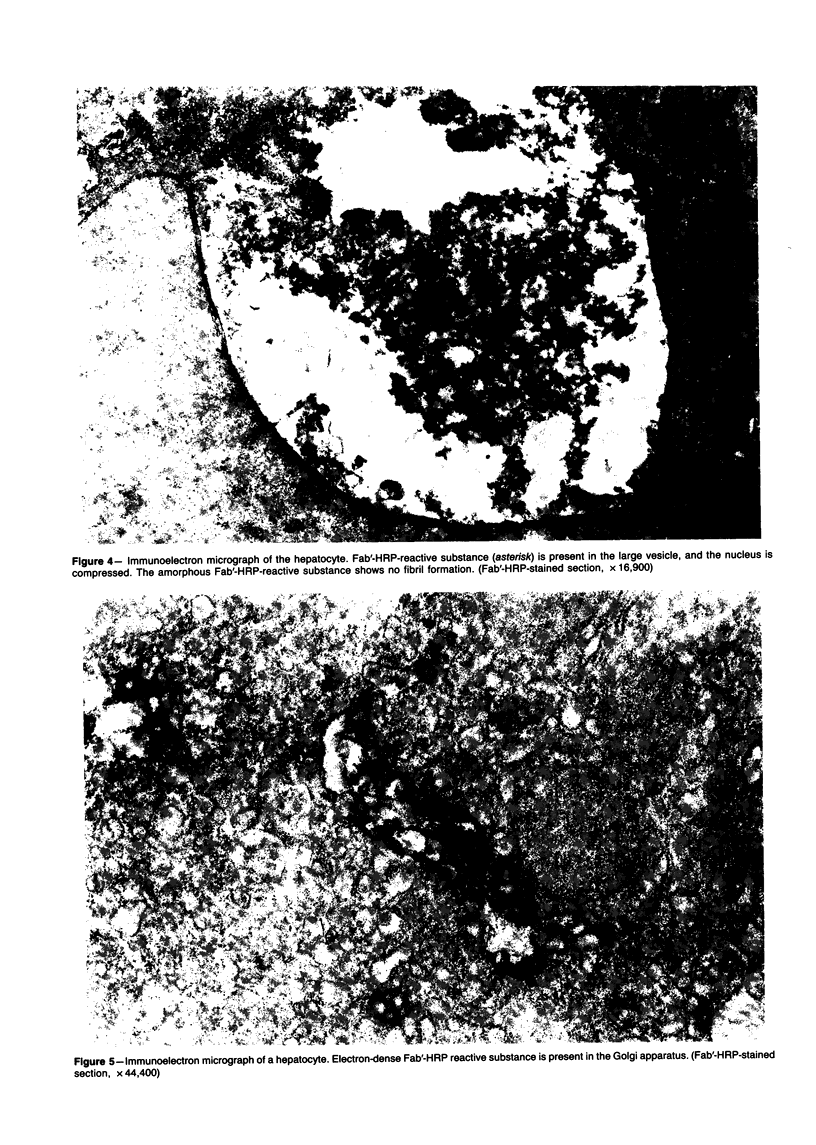
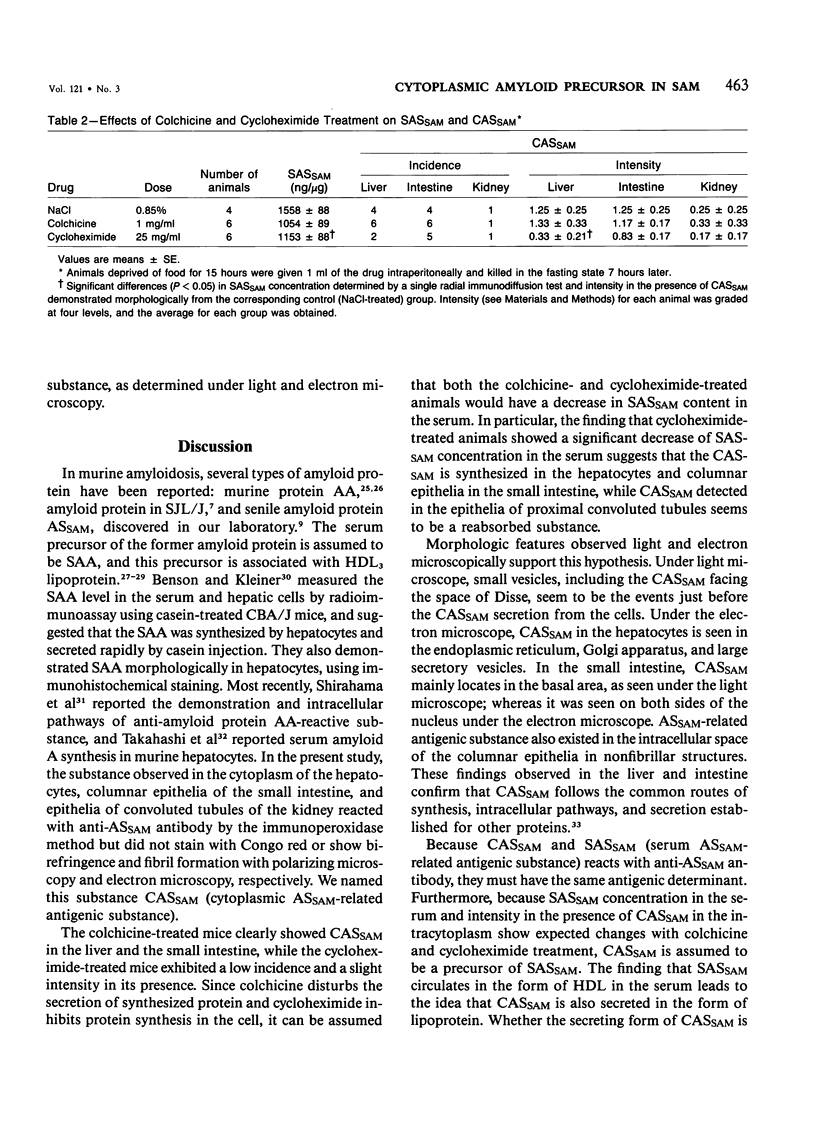
Murine senile amyloid protein identified in the senescence-accelerated mouse (SAM) was called ASSAM, and the ASSAM-related antigenic substance was detected in the cytoplasm of hepatocytes, columnar epithelia of the small intestine, and epithelia of the proximal convoluted tubules of the kidney, with the use of an immunoperoxidase method. This substance, called CASSAM (cytoplasmic ASSAM-related antigenic substance), did not stain positively with Congo red nor fibril structure, as determined under an electron microscope. As the ASSAM (senile amyloid) deposition increased with advancing age, CASSAM observed in hepatocytes and columnar epithelia decreased both in SAM-P and SAM-R strains. In the liver of the SAM-P strain in particular, the incidence and intensity in deposition of ASSAM increased rapidly from 5 months of age; on the other hand, CASSAM observed in the hepatocytes decreased rapidly at about the same time. Cycloheximide-treated animals showed a significantly low concentration of SASSAM (serum ASSAM-related antigenic substance) and also a low incidence and intensity of CASSAM observed in the cytoplasm of the hepatocytes and epithelia of the small intestine. In colchicine-treated animals, SASSAM concentration was slightly lower, and the severity of CASSAM observed in the cytoplasm was slightly higher, in the liver and kidney, as compared with control values. CASSAM is assumed to be synthesized in the cytoplasm of the cell and to be secreted alone or in the lipoprotein form into the serum. This CASSAM or lipoprotein including CASSAM is perhaps a constituent of SASSAM (CASSAM is assumed to include apoSASSAM) and the hepatocytes and intestinal mucosal epithelia are possible production sites of apoSASSAM.
Full text
PDF




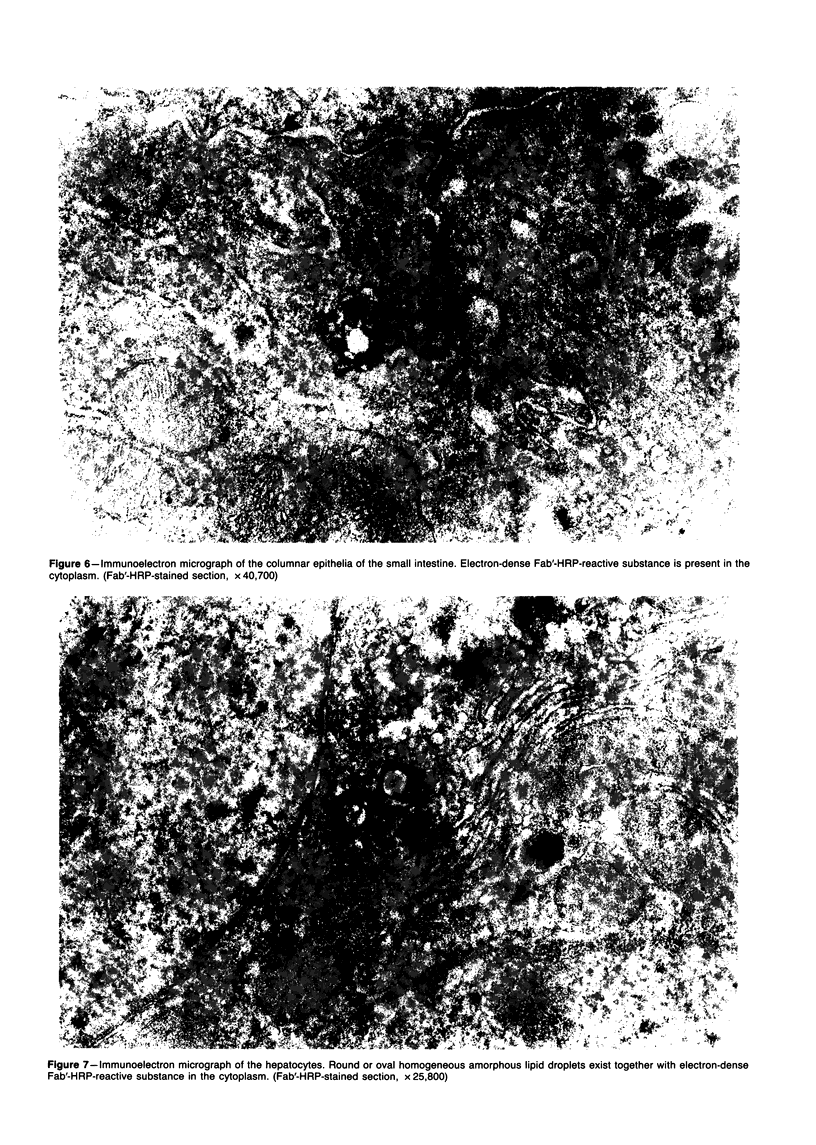
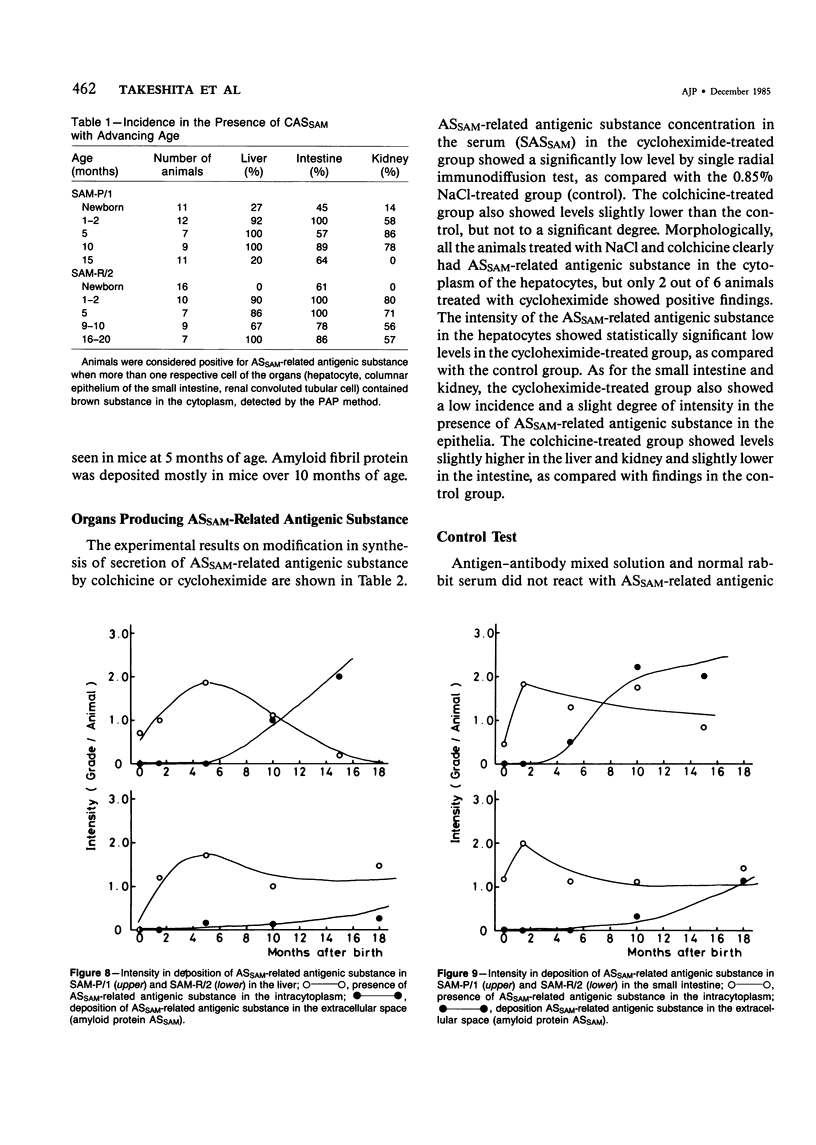


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Kleiner E. Synthesis and secretion of serum amyloid protein A (SAA) by hepatocytes in mice treated with casein. J Immunol. 1980 Feb;124(2):495–499. [PubMed] [Google Scholar]
- Briccetti A. B., Cathcart E. S., Cohen A. S. Casein-induced experimental amyloidosis. 2. Lymphocyte transformation in preamyloidotic and amyloidotic guinea pigs. Acta Pathol Microbiol Scand Suppl. 1972;233:162–166. [PubMed] [Google Scholar]
- Eriksen N., Ericsson L. H., Pearsall N., Lagunoff D., Benditt E. P. Mouse amyloid protein AA: Homology with nonimmunoglobulin protein of human and monkey amyloid substance. Proc Natl Acad Sci U S A. 1976 Mar;73(3):964–967. doi: 10.1073/pnas.73.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara S., Balow J. E., Costa J. C., Glenner G. G. Identification and classification of amyloid in formalin-fixed, paraffin-embedded tissue sections by the unlabeled immunoperoxidase method. Lab Invest. 1980 Oct;43(4):358–365. [PubMed] [Google Scholar]
- Glenner G. G., Page D., Isersky C., Harada M., Cuatrecasas P., Eanes E. D., DeLellis R. A., Bladen H. A., Keiser H. R. Murine amyloid fibril protein: isolation, purification and characterization. J Histochem Cytochem. 1971 Jan;19(1):16–28. doi: 10.1177/19.1.16. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Hashimoto K., Honma A., Takeshita S., Hosokawa M., Yasuhira K., Takeda T. Isolation and characterization of senile amyloid--related antigenic substance (SASSAM) from mouse serum. Apo SASSAM is a low molecular weight apoprotein of high density lipoprotein. J Exp Med. 1983 Nov 1;158(5):1600–1614. doi: 10.1084/jem.158.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Honma A., Takeshita S., Hashimoto K., Hosokawa M., Yasuhira K., Takeda T. Systemic senile amyloid in senescence-accelerated mice. A unique fibril protein demonstrated in tissues from various organs by the unlabeled immunoperoxidase method. Lab Invest. 1983 Feb;48(2):231–240. [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Honma A., Toda K., Takeshita S., Matsushita M., Yonezu T., Hosokawa M., Takeda T. Age-related changes of serum apoprotein SASSAM, apoprotein A-I and low-density lipoprotein levels in senescence accelerated mouse (SAM). Mech Ageing Dev. 1984 Aug;26(2-3):311–326. doi: 10.1016/0047-6374(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Secretion of serum amyloid protein and assembly of serum amyloid protein-rich high density lipoprotein in primary mouse hepatocyte culture. J Biol Chem. 1982 Sep 10;257(17):10518–10522. [PubMed] [Google Scholar]
- Hosokawa M., Kasai R., Higuchi K., Takeshita S., Shimizu K., Hamamoto H., Honma A., Irino M., Toda K., Matsumura A. Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech Ageing Dev. 1984 Jul;26(1):91–102. doi: 10.1016/0047-6374(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Hosokawa M., Takeshita S., Higuchi K., Shimizu K., Irino M., Toda K., Honma A., Matsumura A., Yasuhira K., Takeda T. Cataract and other ophthalmic lesions in senescence accelerated mouse (SAM). Morphology and incidence of senescence associated ophthalmic changes in mice. Exp Eye Res. 1984 Feb;38(2):105–114. doi: 10.1016/0014-4835(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Isersky C., Page D. L., Cuatrecasas P., DeLellis R. A., Glenner G. G. Murine amyloidosis: immunologic characterization of amyloid fibril protein. J Immunol. 1971 Dec;107(6):1690–1698. [PubMed] [Google Scholar]
- JANIGAN D. T. EXPERIMENTAL AMYLOIDOSIS: STUDIES WITH A MODIFIED CASEIN METHOD, CASEIN HYDROLYSATE AND GELATIN. Am J Pathol. 1965 Jul;47:159–171. [PMC free article] [PubMed] [Google Scholar]
- Livni N., Laufer A., Levo Y. Demonstration of amyloid in murine and human secondary amyloidosis by the immunoperoxidase technique. J Pathol. 1980 Dec;132(4):343–348. doi: 10.1002/path.1711320405. [DOI] [PubMed] [Google Scholar]
- Matsumura A., Higuchi K., Shimizu K., Hosokawa M., Hashimoto K., Yasuhira K., Takeda T. A novel amyloid fibril protein isolated from senescence-accelerated mice. Lab Invest. 1982 Sep;47(3):270–275. [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Ram J. S., DeLellis R. A., Glenner G. G. Amyloid. 8. On strain variability in experimental murine amyloidosis. Proc Soc Exp Biol Med. 1969 Feb;130(2):462–464. doi: 10.3181/00379727-130-33580. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S., Eastcott J. W., Skinner M., Benson M., Shirahama T. The SJL/J mouse: a new model for spontaneous age-associated amyloidosis. I. Morphologic and immunochemical aspects. Lab Invest. 1976 Jul;35(1):47–54. [PubMed] [Google Scholar]
- Shimizu K., Ishii M., Yamamuro T., Takeshita S., Hosokawa M., Takeda T. Amyloid deposition in intervertebral discs of senescence-accelerated mouse. Arthritis Rheum. 1982 Jun;25(6):710–712. doi: 10.1002/art.1780250618. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Kasai R., Yamamuro T., Hosokawa M., Takeshita S., Takeda T. Amyloid deposition in the articular structures of AKR senescent mice. Arthritis Rheum. 1981 Dec;24(12):1540–1543. doi: 10.1002/art.1780241213. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Immunocytochemical study of hepatocyte synthesis of amyloid AA. Demonstration of usual site of synthesis and intracellular pathways but unusual retention on the surface membrane. Am J Pathol. 1985 Jan;118(1):108–115. [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Shirahama T., Benson M. D., Cohen A. S. Murine amyloid protein AA in casein-induced experimental amyloidosis. Lab Invest. 1977 Apr;36(4):420–427. [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yokota T., Yamashita Y., Ishihara T., Uchino F. Ultrastructural evidence for the synthesis of serum amyloid A protein by murine hepatocytes. Lab Invest. 1985 Feb;52(2):220–223. [PubMed] [Google Scholar]
- Takeda T., Hosokawa M., Takeshita S., Irino M., Higuchi K., Matsushita T., Tomita Y., Yasuhira K., Hamamoto H., Shimizu K. A new murine model of accelerated senescence. Mech Ageing Dev. 1981 Oct;17(2):183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Hosokawa M., Irino M., Higuchi K., Shimizu K., Yasuhira K., Takeda T. Spontaneous age-associated amyloidosis in senescence-accelerated mouse (SAM). Mech Ageing Dev. 1982 Sep;20(1):13–23. doi: 10.1016/0047-6374(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Westermark P., Sletten K., Naeser P., Natvig J. B. Characterization of amyloid of ageing obese-hyperglycaemic mice and their lean littermates. Scand J Immunol. 1979;9(2):193–196. doi: 10.1111/j.1365-3083.1979.tb02722.x. [DOI] [PubMed] [Google Scholar]