Abstract
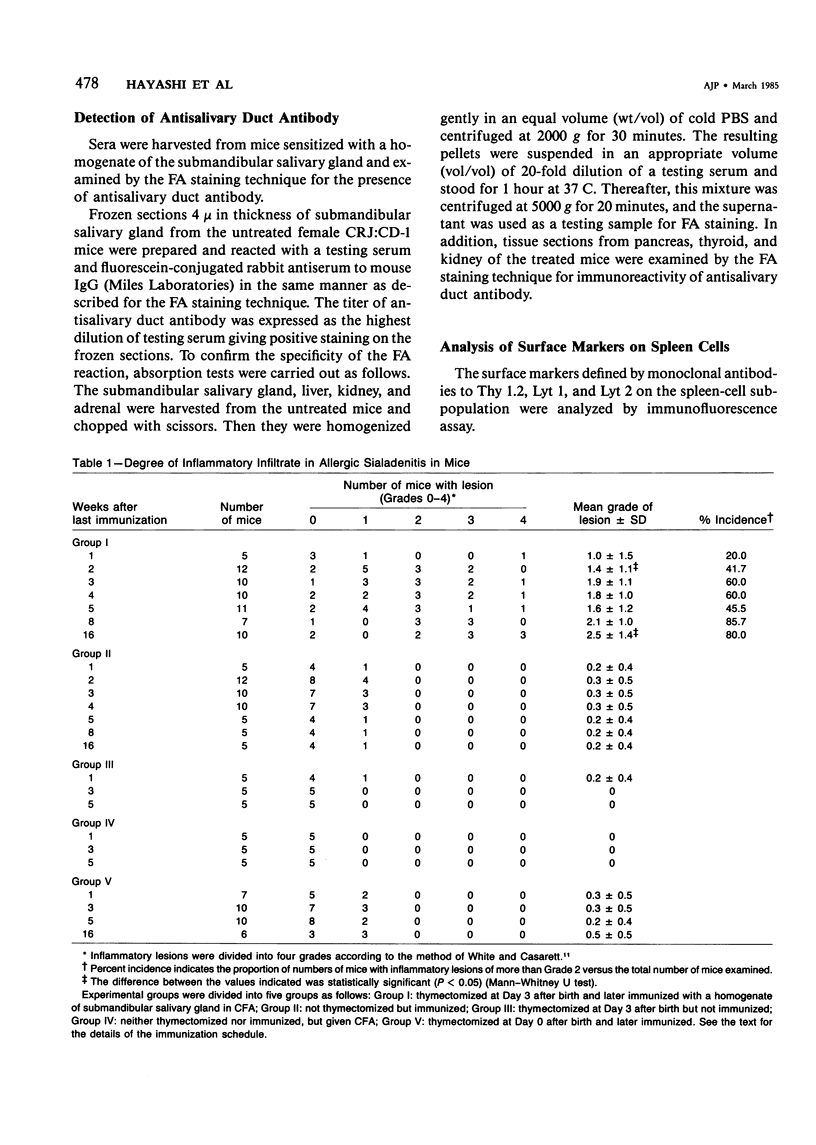
This article reports that sialadenitis developed in female CRJ:CD-1 mice thymectomized 3 days after birth and later immunized with a homogenate of the submandibular salivary gland emulsified with complete Freund's adjuvant. Significant inflammatory changes did not develop in various control groups, including animals thymectomized at Day 3 but not immunized and animals not thymectomized on the day of birth but immunized. Because a more marked decrease of Lyt 2+ cells was found in mice thymectomized on Day 3 after birth than in neonatally thymectomized mice, thymectomy at 3 days of age is more effective for the induction of sialadenitis, presumably by markedly decreasing a population of suppressor T cells. The lesions observed in mice with sialadenitis were mostly composed of small and medium-sized lymphocytes stained by anti-Thy 1.2 and Lyt 2 antibodies and in later stages by immunoglobulin-containing cells in the periphery of inflammatory lesions.
Full text
PDF

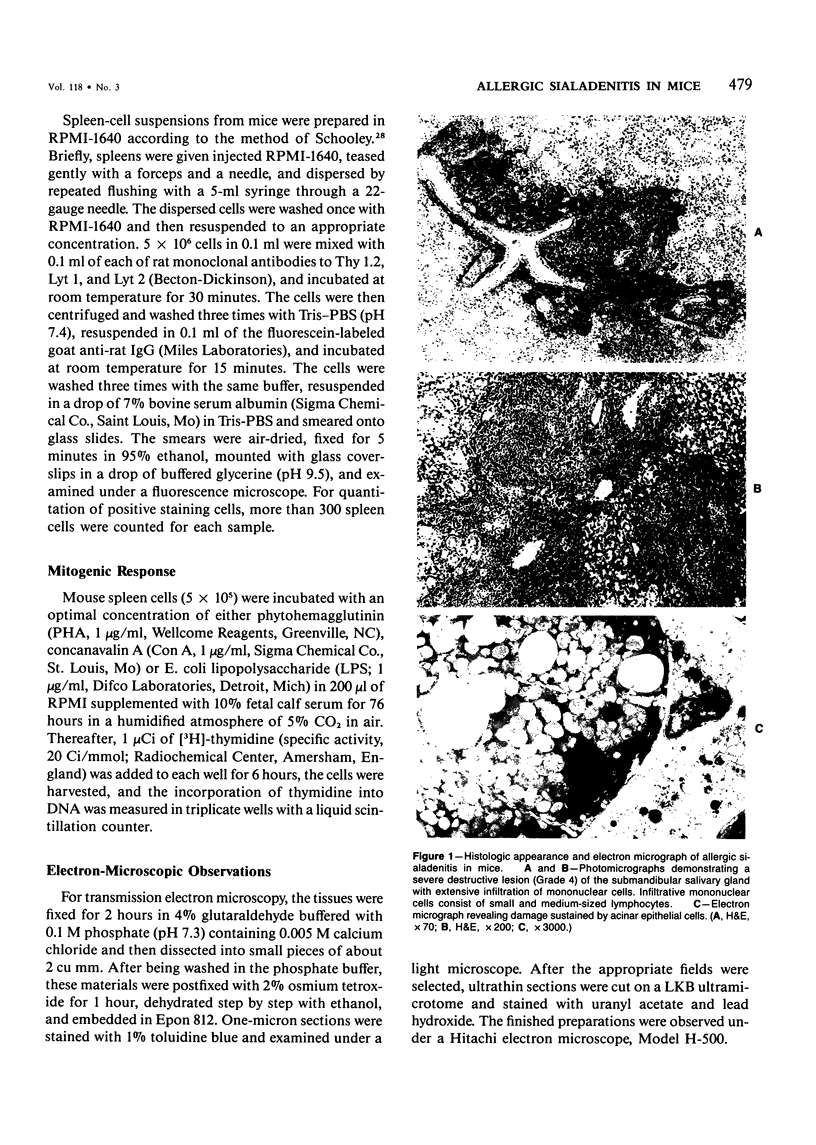
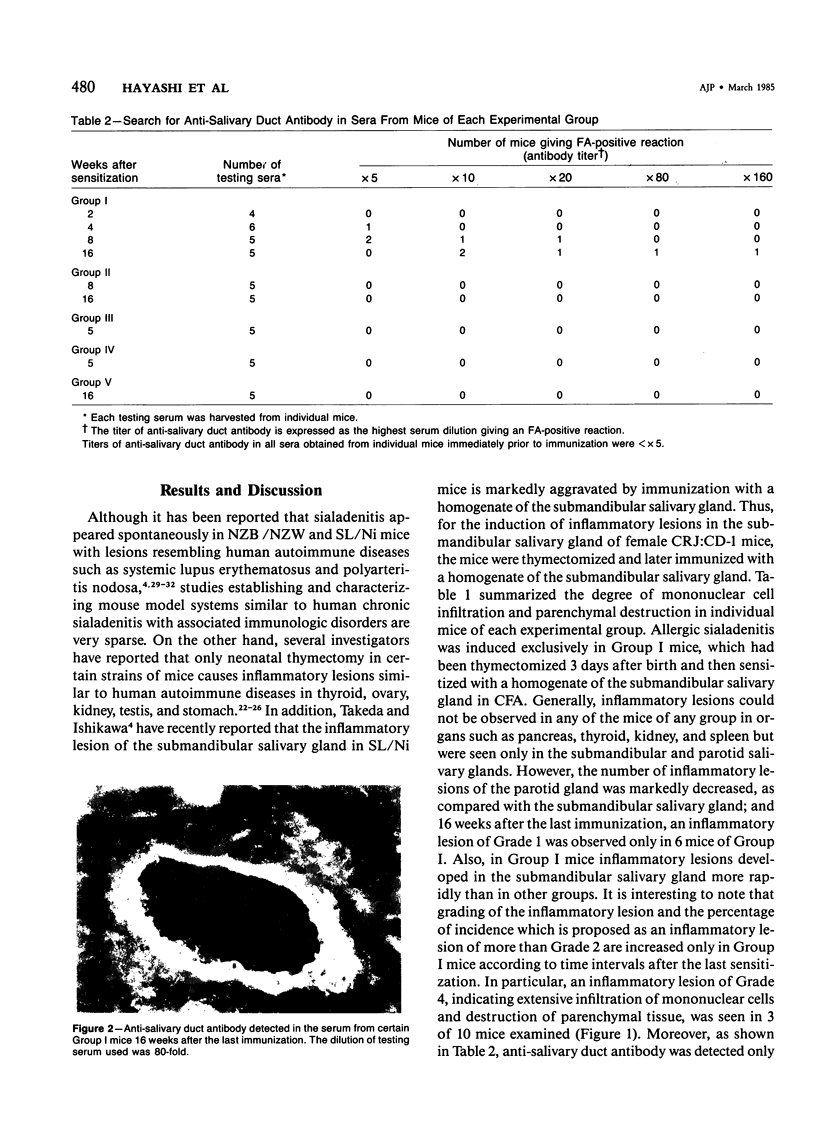
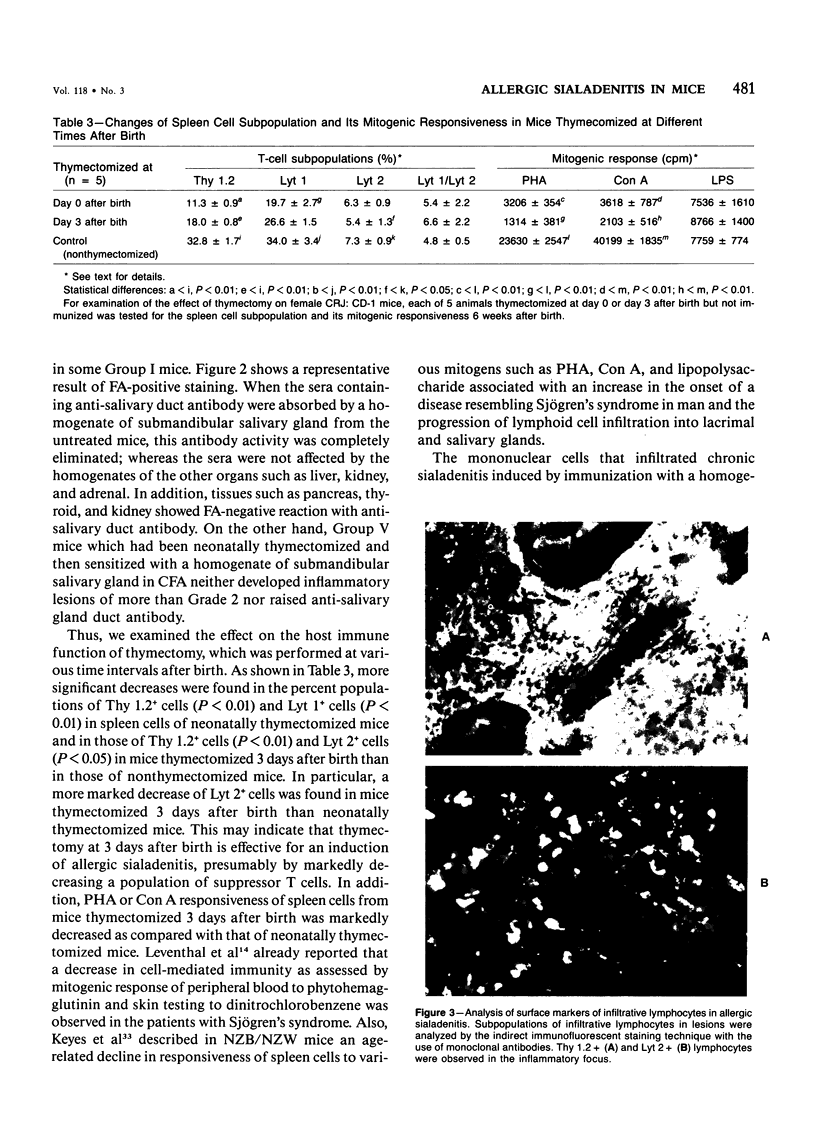
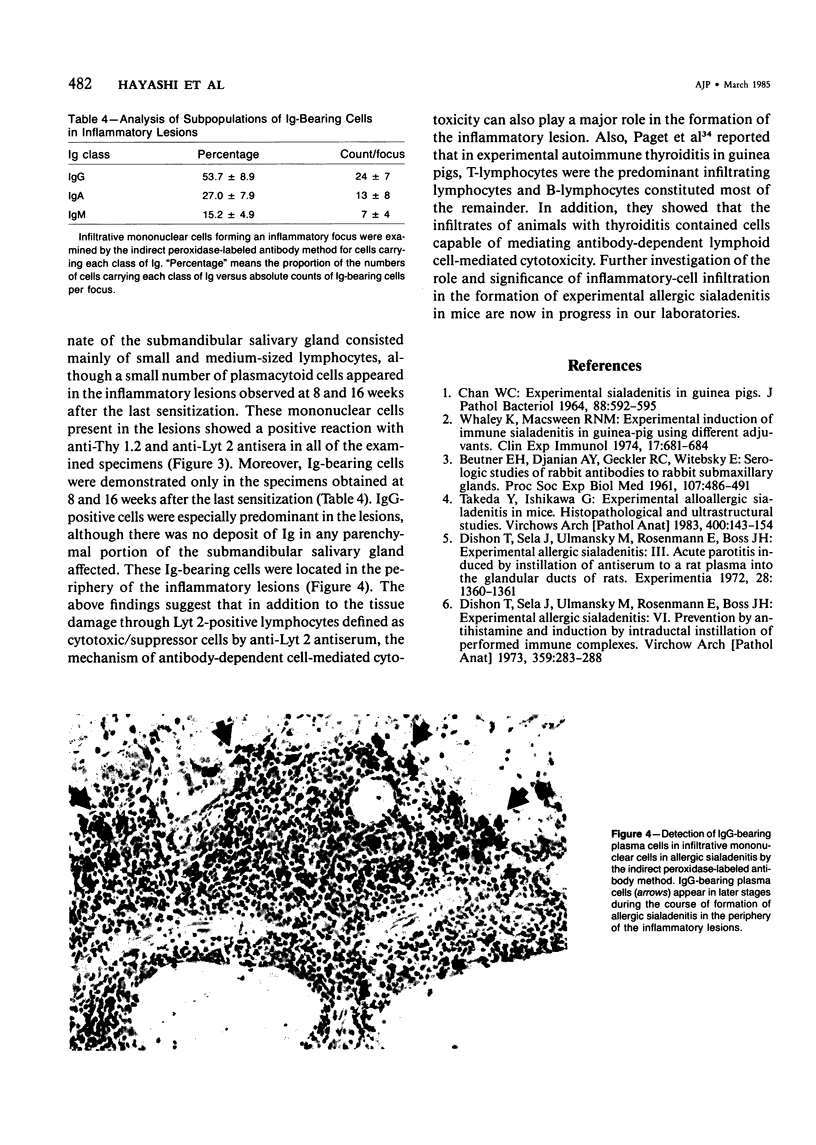

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss J. H., Rosenmann E., Sela J. Experimental allergic sialoadenitis. X. Chronic destructive parotitis induced in immunized rats by daily intraductal challenges with antigen. J Oral Pathol. 1977 Mar;6(2):96–105. doi: 10.1111/j.1600-0714.1977.tb01636.x. [DOI] [PubMed] [Google Scholar]
- CHAN W. C. EXPERIMENTAL SIALO-ADENITIS IN GUINEA-PIGS. J Pathol Bacteriol. 1964 Oct;88:592–595. doi: 10.1002/path.1700880227. [DOI] [PubMed] [Google Scholar]
- Carlsö B., Ostberg Y. Ultrastructural observations on the parotitis autoimmunica in the NZB/NZW hybrid mice. Acta Otolaryngol. 1978 Mar-Apr;85(3-4):298–306. doi: 10.3109/00016487809111939. [DOI] [PubMed] [Google Scholar]
- Dishon T., Sela J., Ulmansky M., Rosenmann E., Boss J. H. Experimental allergic sialoadenitis. VI. Prevention by antihistamine and induction by intraductal instillation of performed immune complexes. Virchows Arch A Pathol Pathol Anat. 1973 Jun 29;359(4):283–288. [PubMed] [Google Scholar]
- Dishon T., Sela Y., Ulmansky M., Rosenmann E., Boss Y. H. Experimental allergic sialoadenitis. 3. Acute parotitis induced by instillation of antiserum to rat plasma into the glandular duct of rats. Experientia. 1972 Nov 15;28(11):1360–1361. doi: 10.1007/BF01965345. [DOI] [PubMed] [Google Scholar]
- Greenspan J. S., Gutman G. A., Weissman I. L., Talal N. Thymus-antigen- and immunoglobulin-positive lymphocytes in tissue infiltrates of NZB/NZW mice. Clin Immunol Immunopathol. 1974 Sep;3(1):16–31. doi: 10.1016/0090-1229(74)90020-8. [DOI] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. THE THYMUS AND AUTOIMMUNE DISEASE. Lancet. 1963 Nov 16;2(7316):1026–1029. doi: 10.1016/s0140-6736(63)90003-5. [DOI] [PubMed] [Google Scholar]
- Kessler H. S. A laboratory model for Sjögren's syndrome. Am J Pathol. 1968 Mar;52(3):671–685. [PMC free article] [PubMed] [Google Scholar]
- Keyes G. G., Vickers R. A., Kersey J. H. Immunopathology of Sjögren-like disease in NZB/HZW mice. J Oral Pathol. 1977 Sep;6(5):288–295. doi: 10.1111/j.1600-0714.1977.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Kojima A., Tanaka-Kojima Y., Sakakura T., Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976 Jun;34(6):550–557. [PubMed] [Google Scholar]
- Lawley T. J., Moutsopoulos H. M., Katz S. I., Theofilopoulos A. N., Chused T. M., Frank M. M. Demonstration of circulating immune complexes in Sjögren's syndrome. J Immunol. 1979 Sep;123(3):1382–1387. [PubMed] [Google Scholar]
- Leventhal B. G., Waldorf D. S., Talal N. Impaired Lymphocyte Transformation and Delayed Hypersensitivity in Sjögren's Syndrome. J Clin Invest. 1967 Aug;46(8):1338–1345. doi: 10.1172/JCI105626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Fauci A. S. Immunoregulation in Sjögren's syndrome: influence of serum factors on T-cell subpopulations. J Clin Invest. 1980 Feb;65(2):519–528. doi: 10.1172/JCI109696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Sakakura T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology. 1971 Sep;89(3):886–893. doi: 10.1210/endo-89-3-886. [DOI] [PubMed] [Google Scholar]
- Paget S. A., McMaster P. R., van Boxel J. A. Experimental autoimmune thyroiditis in the guinea pig: characterization of infiltrating lymphocyte populations. J Immunol. 1976 Dec;117(6):2267–2269. [PubMed] [Google Scholar]
- Rieke W. O. Lymphocytes from thymectomized rats: immunologic, proliferative, and metabolic properties. Science. 1966 Apr 22;152(3721):535–538. doi: 10.1126/science.152.3721.535. [DOI] [PubMed] [Google Scholar]
- Rosenmann E., Sela J., Ulmansky M., Dishon T., Boss J. H. Experimental allergic sialoadenitis. II. Induction of acute sialoadenitis in rats by locally administered basement membrane antibodies. Oral Surg Oral Med Oral Pathol. 1974 Apr;37(4):566–575. doi: 10.1016/0030-4220(74)90288-6. [DOI] [PubMed] [Google Scholar]
- Sela J., Bab J. A., Dishon T., Rosenmann E., Boss J. H. Experimental allergic sialoadenitis. VII. Reactivity of the parotid gland to antigenic challenge in passively immunized rats. J Oral Pathol. 1975 Jul;4(1):11–18. doi: 10.1111/j.1600-0714.1975.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Sela J., Dishon T., Rosenmann E., Ulmansky M., Boss J. H. Experimental allergic sialoadenitis. V. Comparison of the response of the parotid gland and synovial membrane to multiple antigenic challanges. J Oral Pathol. 1973;2(1):7–15. doi: 10.1111/j.1600-0714.1973.tb01669.x. [DOI] [PubMed] [Google Scholar]
- Sharawy M., White S. C. Morphometric and fine structural study of experimental autoallergic sialadenitis of rat submandibular glands. Virchows Arch B Cell Pathol. 1978 Oct 16;28(3):255–273. doi: 10.1007/BF02889075. [DOI] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981 Nov;46(2):425–434. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980 Jun;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ishikawa G. Experimental autoallergic sialadenitis in mice. Histopathological and ultrastructural studies. Virchows Arch A Pathol Anat Histopathol. 1983;400(2):143–154. doi: 10.1007/BF00585496. [DOI] [PubMed] [Google Scholar]
- Ulmansky M., Dishon T., Rosenmann E., Sela J., Boss J. H. Experimental allergic sialoadenitis. 4. Studies on the etiopathogenetic mechanisms of the acute and chronic inflammatory process. Isr J Med Sci. 1972 Nov;8(11):1791–1798. [PubMed] [Google Scholar]
- Welch P., Rose N. R., Kite J. H., Jr Neonatal thymectomy increases spontaneous autoimmune thyroiditis. J Immunol. 1973 Feb;110(2):575–577. [PubMed] [Google Scholar]
- Whaley K., Macsween R. N. Experimental induction of immune sialadenitis in guinea-pigs using different adjuvants. Clin Exp Immunol. 1974 Aug;17(4):681–684. [PMC free article] [PubMed] [Google Scholar]
- White S. C., Casarett G. W. Induction of experimental autoallergic sialadenitis. J Immunol. 1974 Jan;112(1):178–185. [PubMed] [Google Scholar]
- Yoshinoya S., McDuffy S., Alarcon-Segovia D., Pope R. M. Detection and partial characterization of immune complexes in patients with rheumatoid arthritis plus Sjogren's syndrome and with Sjogren's syndrome alone. Clin Exp Immunol. 1982 May;48(2):339–347. [PMC free article] [PubMed] [Google Scholar]
- Yunis E. J., Teague P. O., Stutman O., Good R. A. Post-thymectomy autoimmune phenomena in mice. II. Morphologic observations. Lab Invest. 1969 Jan;20(1):46–61. [PubMed] [Google Scholar]
- Zinneman H. H., Caperton E. Cryoglobulinemia in a patient with Sjögren's syndrome, and factors of cryoprecipitation. J Lab Clin Med. 1977 Mar;89(3):483–487. [PubMed] [Google Scholar]