Abstract
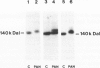
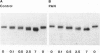
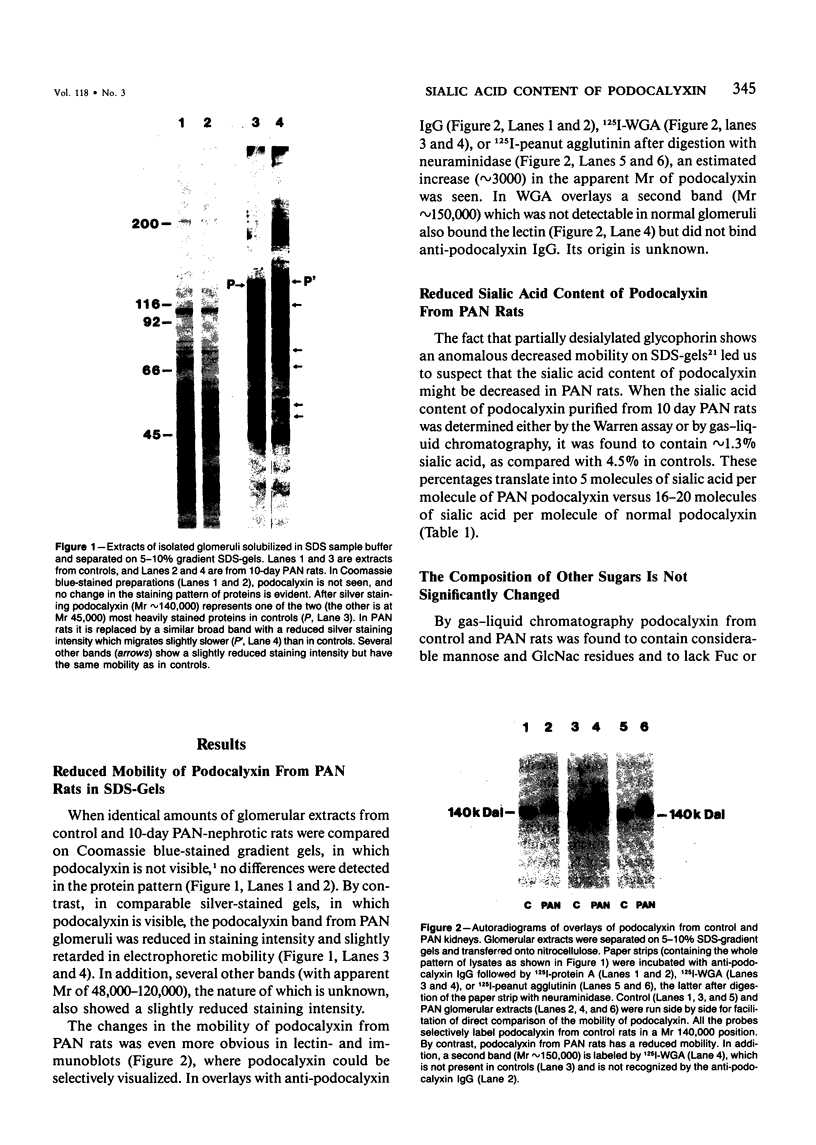
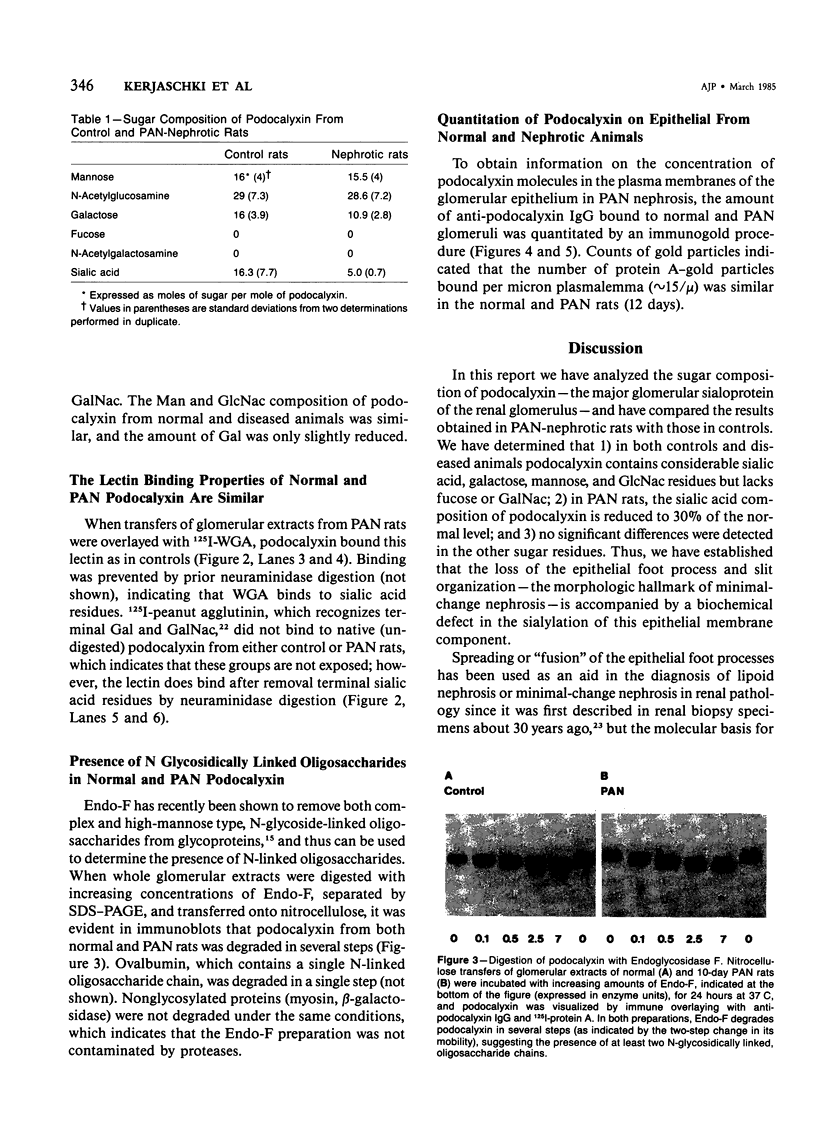
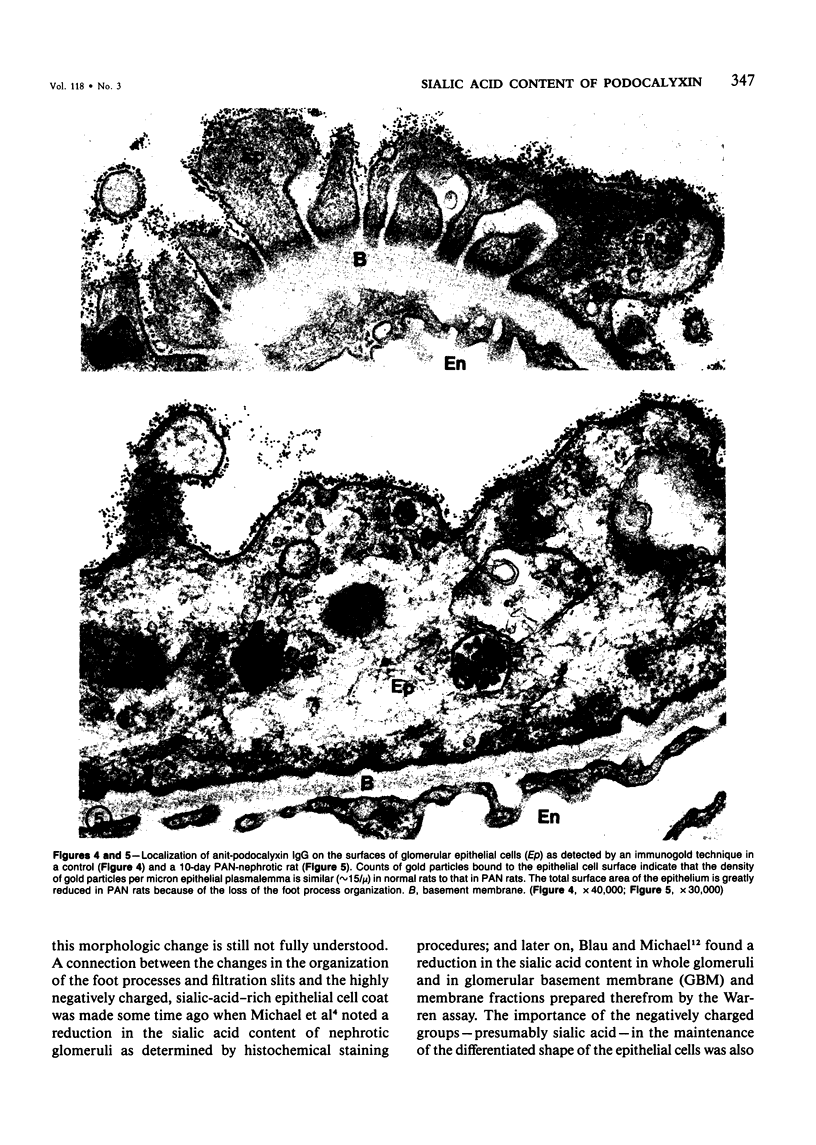
In this study the sugar composition of podocalyxin was determined in puromycin aminonucleoside-treated (PAN) rats and controls. Podocalyxin from both control and PAN rats bound 125I-WGA and 125I-peanut lectin (the latter only after neuraminidase treatment) on nitrocellulose transfers. Purified podocalyxin from both control and PAN rats was found to contain sialic acid, Gal, GlcNac, and Man but lacked Fuc and GalNac by gas-liquid chromatography. In PAN rats the sialic acid content of podocalyxin was reduced from 4.5% to 1.5%, whereas the concentration of the other sugars (with the possible exception of Gal) was similar to that of controls. The density of podocalyxin on the epithelial cell surface was estimated after immunogold labeling with anti-podocalyxin IgG, and no differences were found between PAN rats and controls. These data indicate that the reduced total glomerular sialic acid content found in PAN is due to the combined effects of the decreased podocyte plasmalemmal surface area and the reduced sialic acid content of podocalyxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Yu R. K. Isolation and characterization of a novel trisialoganglioside, GT1a, from human brain. J Biol Chem. 1977 Sep 25;252(18):6247–6250. [PubMed] [Google Scholar]
- Andrews P. M. Glomerular epithelial alterations resulting from sialic acid surface coat removal. Kidney Int. 1979 Apr;15(4):376–385. doi: 10.1038/ki.1979.49. [DOI] [PubMed] [Google Scholar]
- Andrews P. M., Stauver M. In vitro incubation and study of kidney glomerular epithelial cells. Kidney Int. 1979 Jan;15(1):80–87. doi: 10.1038/ki.1979.11. [DOI] [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S., Kochwa S. Structure of the carbohydrate units of IgE immunoglobulin. I. Over-all composition, glycopeptide isolation, and structure of the high mannose oligosaccharide unit. J Biol Chem. 1974 Mar 25;249(6):1889–1896. [PubMed] [Google Scholar]
- Blau E. B., Haas J. E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973 Apr;28(4):477–481. [PubMed] [Google Scholar]
- Blau E. B., Michael A. F. Rat glomerular glycoprotein composition and metabolism in aminonucleoside nephrosis. Proc Soc Exp Biol Med. 1972 Oct;141(1):164–172. doi: 10.3181/00379727-141-36737. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Bridges C. R., Myers B. D., Brenner B. M., Deen W. M. Glomerular charge alterations in human minimal change nephropathy. Kidney Int. 1982 Dec;22(6):677–684. doi: 10.1038/ki.1982.229. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., VERNIER R. L., GOOD R. A. Studies on familial nephrosis. II. Glomerular changes observed with the electron microscope. Am J Pathol. 1957 Jul-Aug;33(4):791–817. [PMC free article] [PubMed] [Google Scholar]
- Faraggiana T., Grishman E. The podocyte cell coat in experimental nephrosis. J Ultrastruct Res. 1981 Mar;74(3):296–306. doi: 10.1016/s0022-5320(81)80120-7. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Role of sialic acid in the mobility of membrane proteins containing O-linked oligosaccharides on polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Eur J Biochem. 1982 Mar 1;122(3):581–586. doi: 10.1111/j.1432-1033.1982.tb06478.x. [DOI] [PubMed] [Google Scholar]
- Hammond K. S., Papermaster D. S. Fluorometric assay of sialic acid in the picomole range: a modification of the thiobarbituric acid assay. Anal Biochem. 1976 Aug;74(2):292–297. doi: 10.1016/0003-2697(76)90210-4. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Pettersson E., Törnroth T., Alfthan O., Linder E., Miettinen A. Lectins as fluorescence microscopic markers for saccharides in the human kidney. Lab Invest. 1981 Nov;45(5):391–399. [PubMed] [Google Scholar]
- Jones D. B. Mucosubstances of the glomerulus. Lab Invest. 1969 Aug;21(2):119–125. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
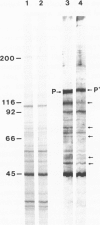
- Kerjaschki D., Sharkey D. J., Farquhar M. G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984 Apr;98(4):1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. J., Dehnel P. J., Oegema T. R., Brown D. M. Alterations in proteoglycan metabolism in the nephrotic syndrome induced by the aminonucleoside of puromycin. Lab Invest. 1984 May;50(5):543–551. [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mohos S. C., Skoza L. Glomerular sialoprotein. Science. 1969 Jun 27;164(3887):1519–1521. doi: 10.1126/science.164.3887.1519. [DOI] [PubMed] [Google Scholar]
- Murata F., Tsuyama S., Suzuki S., Hamada H., Ozawa M., Muramatsu T. Distribution of glycoconjugates in the kidney studied by use of labeled lectins. J Histochem Cytochem. 1983 Jan;31(1A):139–144. [PubMed] [Google Scholar]
- Reeves W. H., Kanwar Y. S., Farquhar M. G. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J Cell Biol. 1980 Jun;85(3):735–753. doi: 10.1083/jcb.85.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W., Caulfield J. P., Farquhar M. G. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978 Aug;39(2):90–100. [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. Isolation and partial characterization of the sialoglycoprotein fraction of murine erythrocyte ghosts. J Cell Biol. 1982 Jun;93(3):583–590. doi: 10.1083/jcb.93.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler M. W., Rennke H. G., Venkatachalam M. A., Cotran R. S. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest. 1977 Jan;36(1):48–61. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]