Abstract
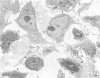
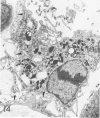
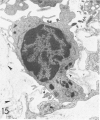
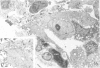
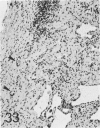
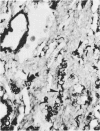
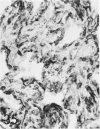
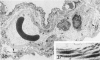
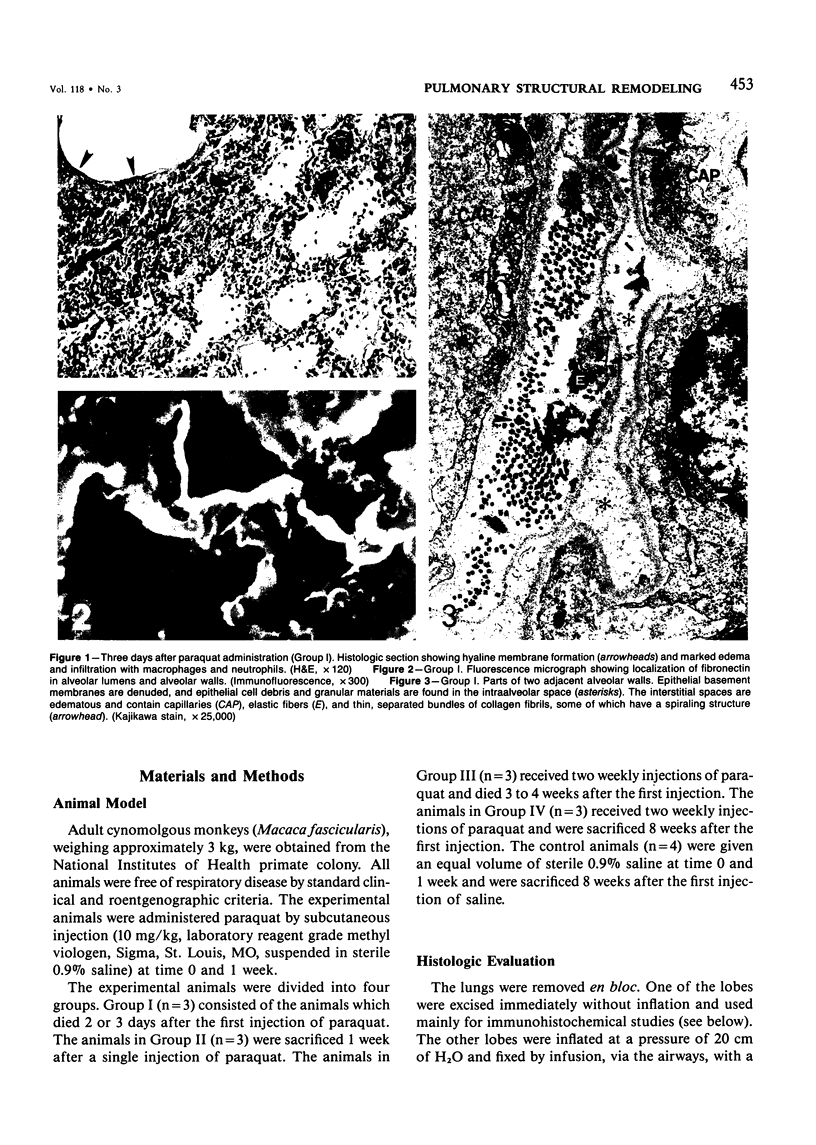
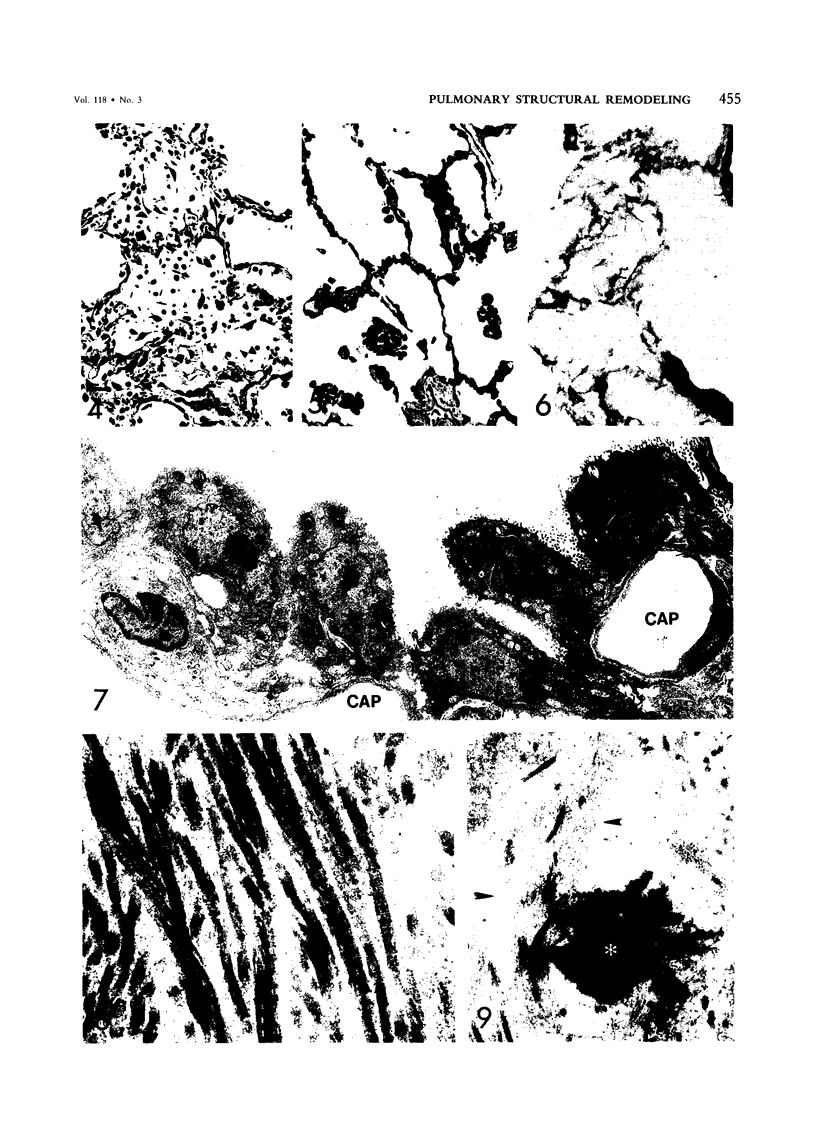
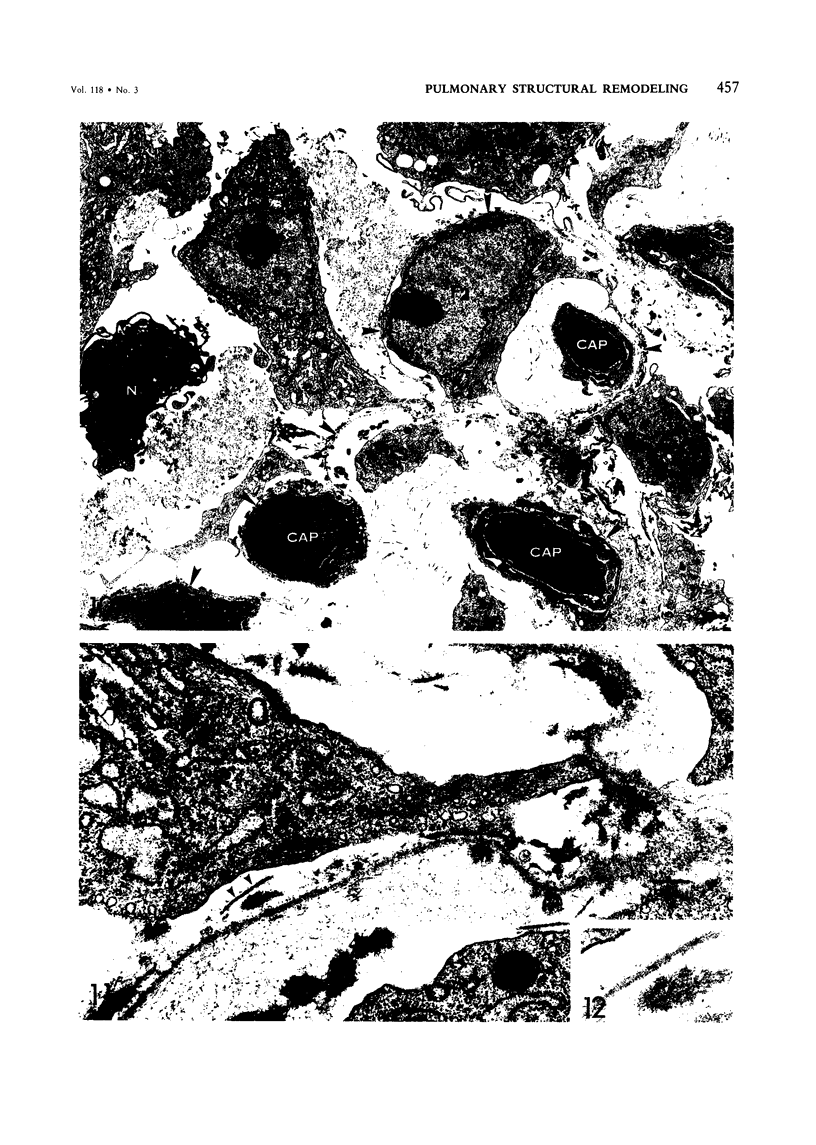
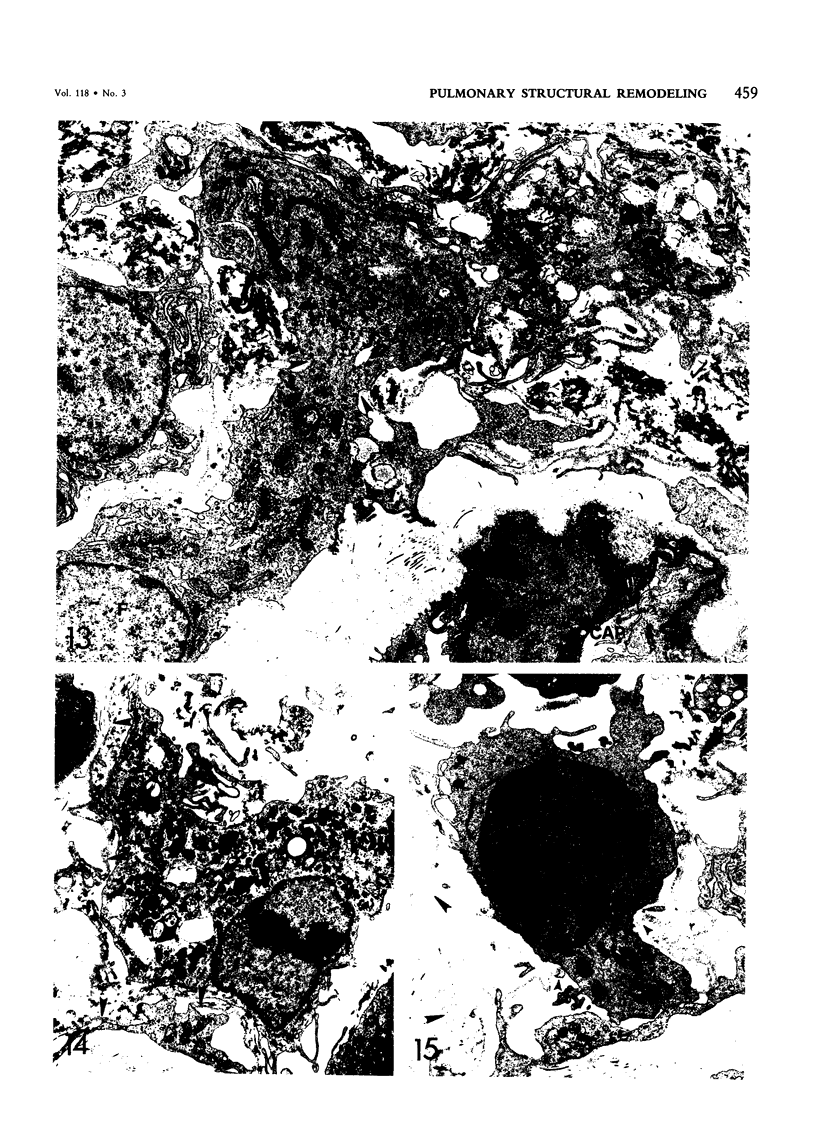
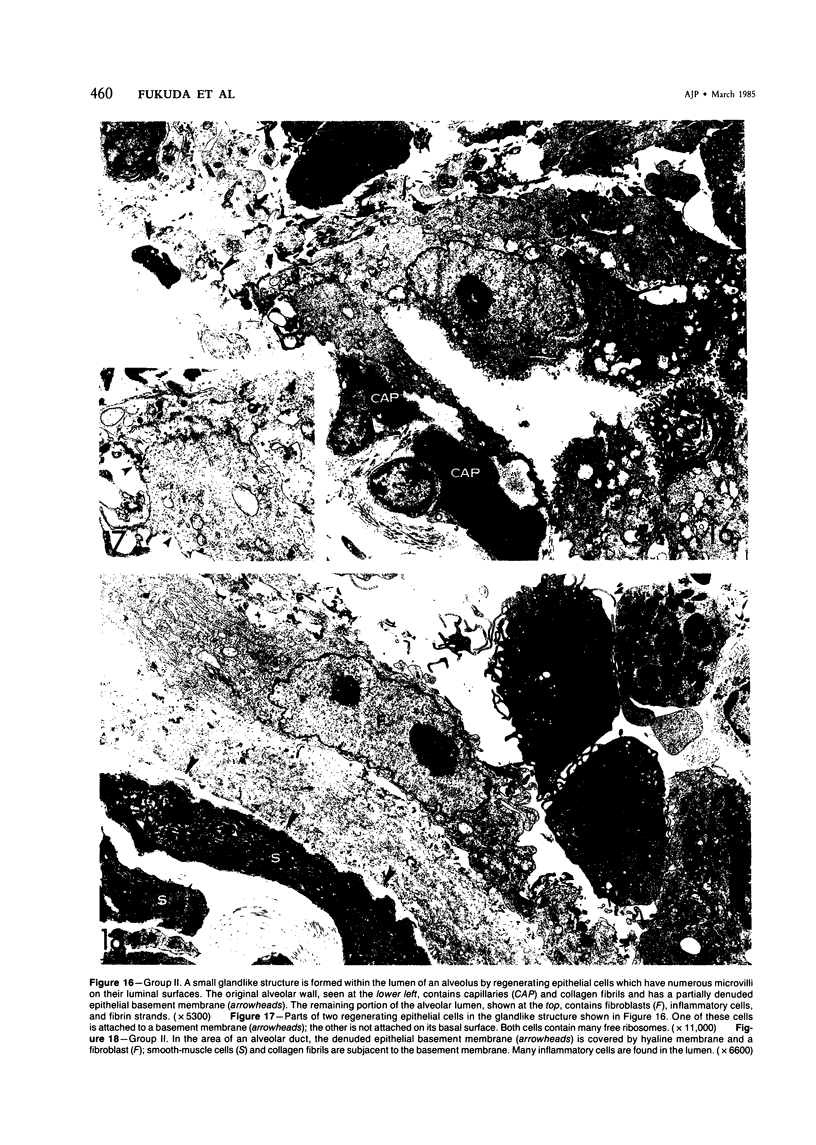
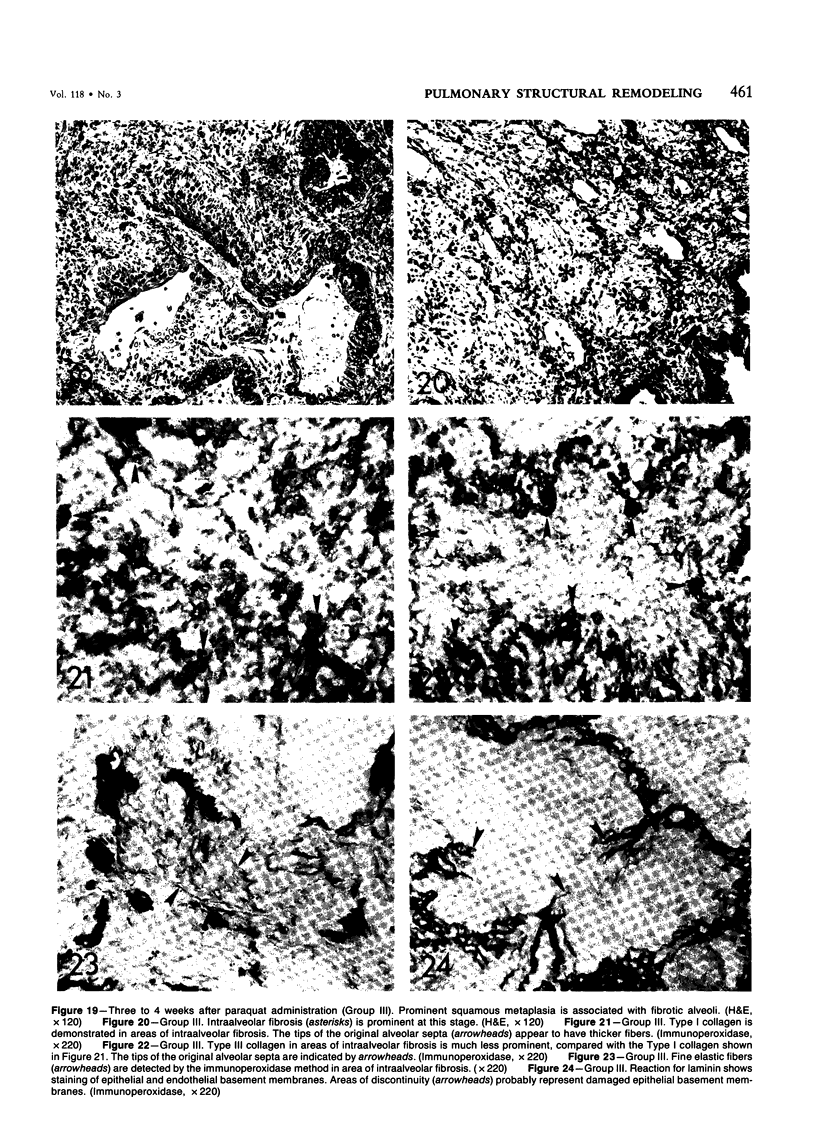
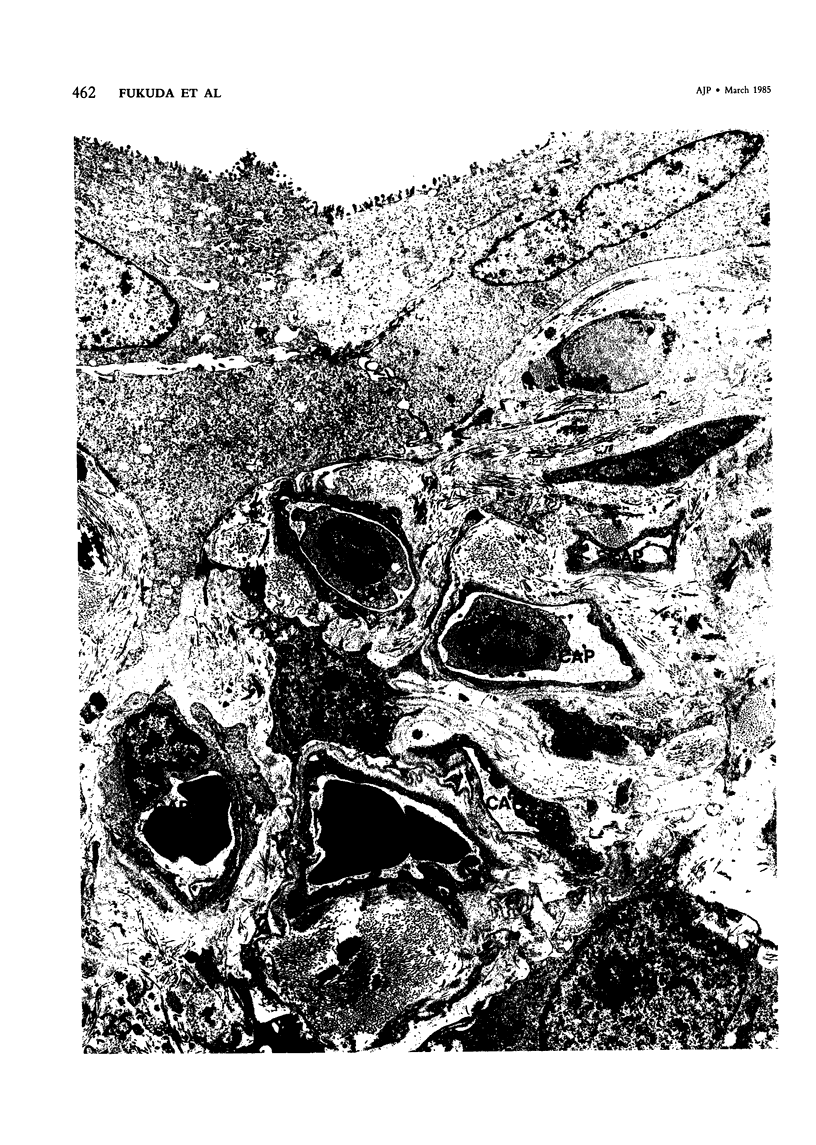
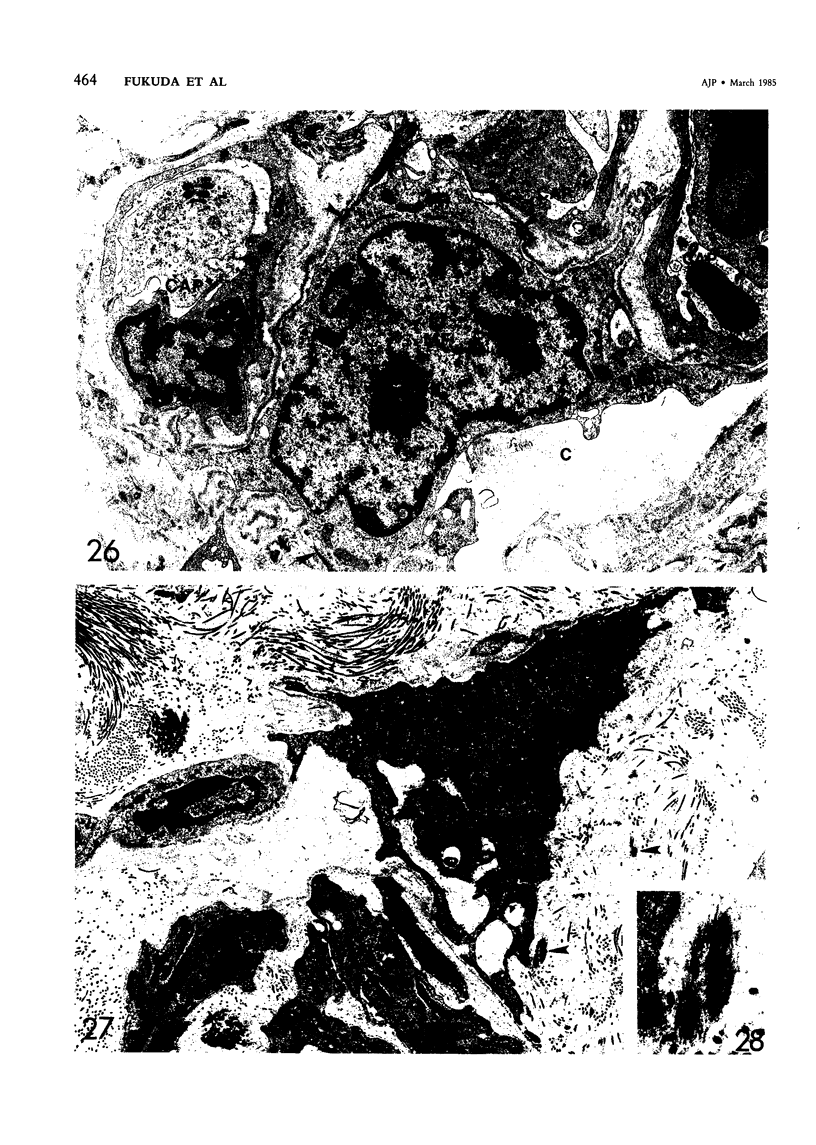
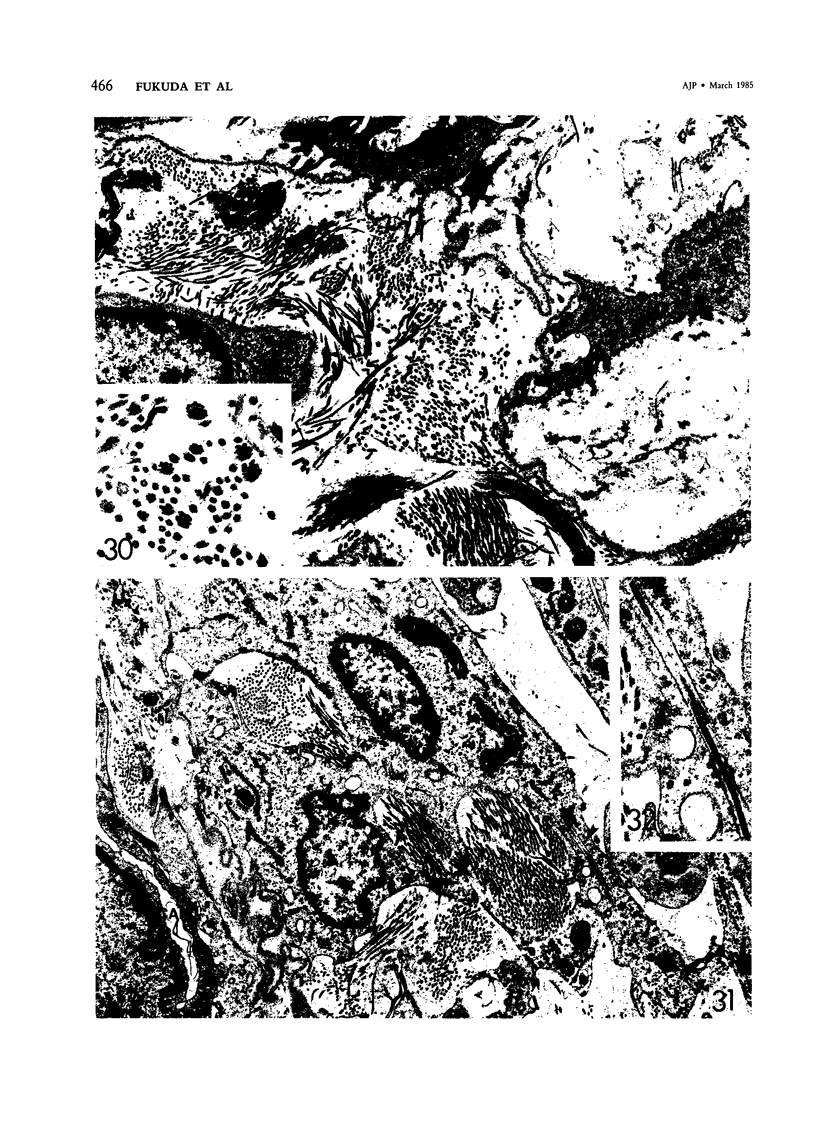
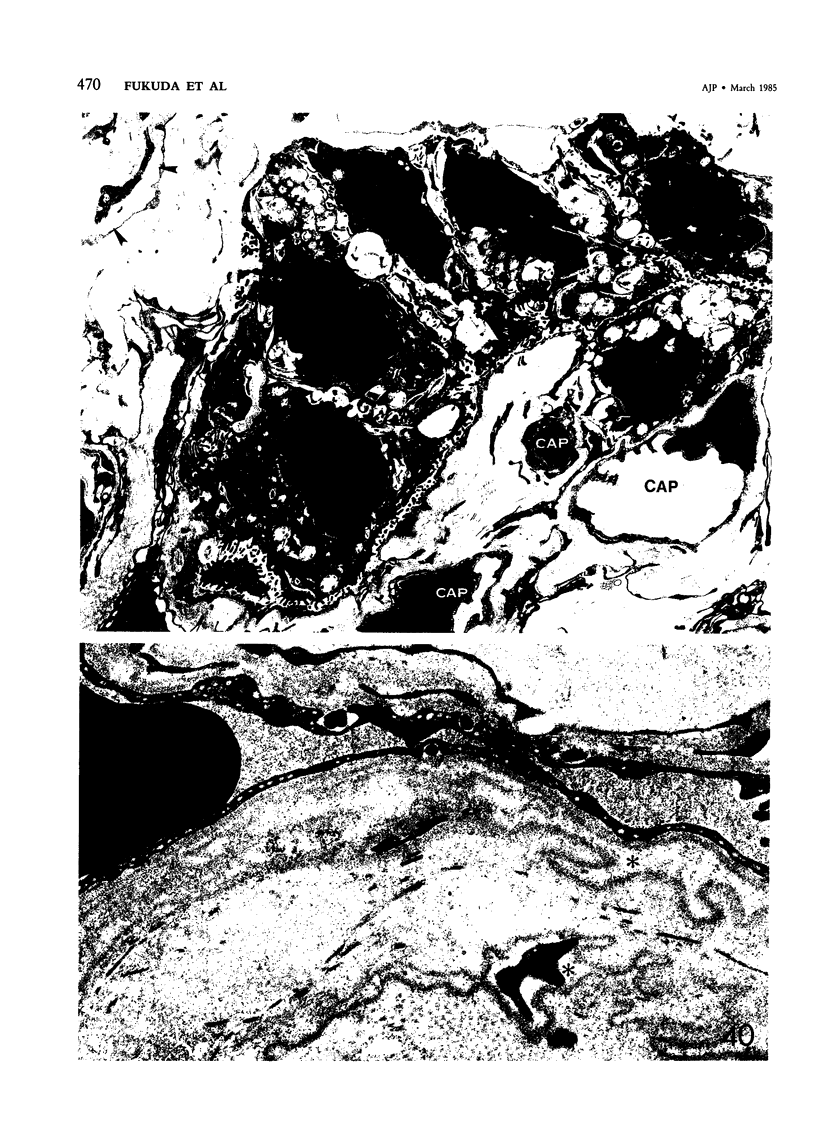
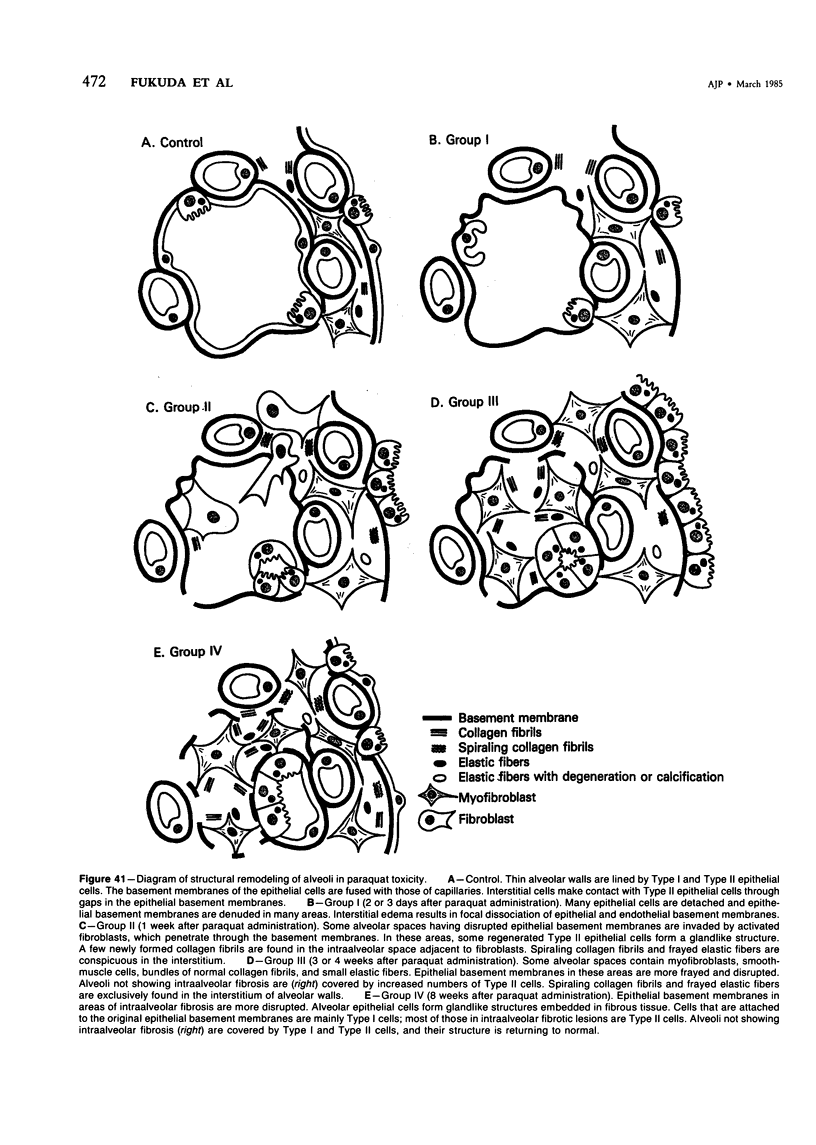
For a study of the evolution of interstitial and intraalveolar fibrosis, ultrastructural and immunohistochemical observations were made of the lungs of 16 cynomolgous monkeys given 1 or 2 injections of 10 mg/kg of paraquat and sacrificed 2 days to 8 weeks later. At 2-3 days, alveolar epithelial cells were denuded in many areas, and fibronectin was conspicuous in alveolar spaces. At 1 week, fibroblasts and inflammatory cells were migrating through gaps in the denuded epithelial basement membranes; Type II cells were regenerating in some areas. At 3-4 weeks, alveoli developing intraalveolar fibrosis contained many myofibroblasts, collagen fibrils, and small elastic fibers; fibrotic alveolar walls were lined by metaplastic squamous cells and bronchiolar epithelial cells. Spiraling collagen fibrils were found in interstitium but not in alveolar spaces, which suggests that they were formed from breakdown of collagen. Newly formed intraalveolar collagen was mainly Type I. At 8 weeks, intraalveolar fibrosis had led to extensive remodeling, with new glandlike alveoli lined by Type II cells; alveoli without intraalveolar fibrosis had more normal architecture. Thus, intraalveolar fibrosis in paraquattreated lung is mediated by intraalveolar migration of interstitial cells, through gaps in the epithelial basement membranes, after epithelial injury. This is followed by connective tissue synthesis on the luminal side of the epithelial basement membrane, by differentiation of interstitial cells into myofibroblasts and smooth-muscle cells, by incorporation of areas of intraalveolar fibrosis into the interstitium, and by coalescence of alveolar walls. Intraalveolar fibrosis is more important than interstitial fibrosis in the structural remodeling that occurs in paraquattreated lung, because it results in obliteration of alveoli, coalescence of alveolar walls, and loss of functional alveolar-capillary units.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Basic pattern of tissue repair in human lungs following unspecific injury. Chest. 1974 Apr;65(Suppl):14S–19S. doi: 10.1378/chest.65.4_supplement.14s. [DOI] [PubMed] [Google Scholar]
- Basset F., Le Crom M., Decroix G. Etude ultrastructurale d'une biopsie pulmonaire au cours d'une maladie des éleveurs d'oiseaux. Pneumopathie interstitielle d'hypersensibilité. Presse Med. 1970 Mar 28;78(15):699–703. [PubMed] [Google Scholar]
- Basset F., Soler P., Marsac J., Corrin B. Pulmonary lymphangiomyomatosis: three new cases studied with electron microscopy. Cancer. 1976 Dec;38(6):2357–2366. doi: 10.1002/1097-0142(197612)38:6<2357::aid-cncr2820380623>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Belton J. C., Crise N., McLaughlin R. F., Jr, Tueller E. E. Ultrastructural alterations in collagen associated with microscopic foci of human emphysema. Hum Pathol. 1977 Nov;8(6):669–677. doi: 10.1016/s0046-8177(77)80095-6. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Steinmann B., Rennard S. I., Crystal R. G. Ascorbate deficiency results in decreased collagen production: under-hydroxylation of proline leads to increased intracellular degradation. Arch Biochem Biophys. 1983 Oct 15;226(2):681–686. doi: 10.1016/0003-9861(83)90338-7. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Adelberg S., Crystal R. G. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983 Dec;97(6):1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchard F. Ultrastrukturelle und lichtmikroskopische Befunde bei drei protrahiert tödlich verlaufenen Paraquatvergiftungen. Pneumonologie. 1974;150(2-4):185–189. doi: 10.1007/BF02179318. [DOI] [PubMed] [Google Scholar]
- Braverman I. M., Fonferko E. Studies in cutaneous aging: I. The elastic fiber network. J Invest Dermatol. 1982 May;78(5):434–443. doi: 10.1111/1523-1747.ep12507866. [DOI] [PubMed] [Google Scholar]
- Brooks R. E. Ultrastructure of lung lesions produced by ingested chemicals. I. Effect of the herbicide paraquat on mouse lung. Lab Invest. 1971 Dec;25(6):536–545. [PubMed] [Google Scholar]
- Cergneux M., Andersen E., Cimasoni G. In vitro breakdown of gingival tissue by elastase from human polymorphonuclear leukocytes. An electron microscopic study. J Periodontal Res. 1982 Mar;17(2):169–182. doi: 10.1111/j.1600-0765.1982.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Clark D. G., McElligott T. F., Hurst E. W. The toxicity of paraquat. Br J Ind Med. 1966 Apr;23(2):126–132. doi: 10.1136/oem.23.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland G. M., Kolín A., Shulman H. S. Fatal pulmonary intra-alveolar fibrosis after paraquat ingestion. N Engl J Med. 1974 Aug 8;291(6):290–292. doi: 10.1056/NEJM197408082910607. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Baum B. J., Bernardo J., Bradley K. H., Bruel S. D., Elson N. A., Fells G. A., Ferrans V. J., Gadek J. E. Cells, collagen and idiopathic pulmonary fibrosis. Lung. 1978;155(3):199–224. doi: 10.1007/BF02730694. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Foidart J. M., Berman J. J., Paglia L., Rennard S., Abe S., Perantoni A., Martin G. R. Synthesis of fibronectin, laminin, and several collagens by a liver-derived epithelial line. Lab Invest. 1980 May;42(5):525–532. [PubMed] [Google Scholar]
- Fukuda Y., Ferrans V. J., Crystal R. G. Development of elastic fibers of nuchal ligament, aorta, and lung of fetal and postnatal sheep: an ultrastructural and electron microscopic immunohistochemical study. Am J Anat. 1984 Aug;170(4):597–629. doi: 10.1002/aja.1001700407. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Ferrans V. J., Crystal R. G. The development of alveolar septa in fetal sheep lung. An ultrastructural and immunohistochemical study. Am J Anat. 1983 Aug;167(4):405–439. doi: 10.1002/aja.1001670402. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Ferrans V. J. The electron microscopic immunohistochemistry of elastase-treated aorta and nuchal ligament of fetal and postnatal sheep. J Histochem Cytochem. 1984 Jul;32(7):747–756. doi: 10.1177/32.7.6376618. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Hunninghake G. W., Fells G. A., Zimmerman R. L., Keogh B. A., Crystal R. G. Evaluation of the protease-antiprotease theory of human destructive lung disease. Bull Eur Physiopathol Respir. 1980;16 (Suppl):27–40. doi: 10.1016/b978-0-08-027379-2.50005-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Greenberg D. B., Reiser K. M., Last J. A. Correlation of biochemical and morphologic manifestations of acute pulmonary fibrosis in rats administered paraquat. Chest. 1978 Oct;74(4):421–425. doi: 10.1378/chest.74.4.421. [DOI] [PubMed] [Google Scholar]
- Haschek W. M., Klein-Szanto A. J., Last J. A., Reiser K. M., Witschi H. Long-term morphologic and biochemical features of experimentally induced lung fibrosis in the mouse. Lab Invest. 1982 Apr;46(4):438–449. [PubMed] [Google Scholar]
- Kajikawa K., Nakanishi I., Yamamura T. The effect of collagenase on the formation of fibrous long spacing collagen aggregates. Lab Invest. 1980 Nov;43(5):410–417. [PubMed] [Google Scholar]
- Kajikawa K., Yamaguchi T., Katsuda S., Miwa A. An improved electron stain for elastic fibers using tannic acid. J Electron Microsc (Tokyo) 1975;24(4):287–289. [PubMed] [Google Scholar]
- Kapanci Y., Assimacopoulos A., Irle C., Zwahlen A., Gabbiani G. "Contractile interstitial cells" in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol. 1974 Feb;60(2):375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kawanami O., Basset F., Barrios R., Lacronique J. G., Ferrans V. J., Crystal R. G. Hypersensitivity pneumonitis in man. Light- and electron-microscopic studies of 18 lung biopsies. Am J Pathol. 1983 Mar;110(3):275–289. [PMC free article] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 1982 Jan;46(1):39–53. [PubMed] [Google Scholar]
- Kelly D. F., Morgan D. G., Darke P. G., Gibbs C., Pearson H., Weaver B. M. Pathology of acute respiratory distress in the dog associated with paraquat poisoning. J Comp Pathol. 1978 Apr;88(2):275–294. [PubMed] [Google Scholar]
- Kuhn C., Yu S. Y., Chraplyvy M., Linder H. E., Senior R. M. The induction of emphysema with elastase. II. Changes in connective tissue. Lab Invest. 1976 Apr;34(4):372–380. [PubMed] [Google Scholar]
- Lillie J. H., MacCallum D. K., Scaletta L. J., Occhino J. C. Collagen structure: evidence for a helical organization of the collagen fibril. J Ultrastruct Res. 1977 Feb;(2):134–143. doi: 10.1016/s0022-5320(77)90025-9. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980 Jul;11(4):353–366. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- Manebe T., Kikkawa Y. Helical structure of human native collagen. Arch Pathol Lab Med. 1976 May;100(5):259–264. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Miller E. J., Damjanov I., Gay S. Laminin-secreting yolk sac carcinoma of the rat. Biochemical and electron immunohistochemical studies. Lab Invest. 1982 Sep;47(3):247–257. [PubMed] [Google Scholar]
- Matthew H., Logan A., Woodruff M. F., Heard B. Paraquat poisoning--lung transplantation. Br Med J. 1968 Sep 28;3(5621):759–763. doi: 10.1136/bmj.3.5621.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Miyazaki N., Takamoto M., Kinjo M., Ishibashi T. Ultrastructural studies of elastase-induced experimental emphysema. Jpn J Exp Med. 1979 Aug;49(4):241–250. [PubMed] [Google Scholar]
- Morris S. M., Stone P. J., Snider G. L., Albright J. T., Franzblau C. Ultrastructural changes in hamster lung four hours to twenty-four days after exposure to elastase. Anat Rec. 1981 Nov;201(3):523–535. doi: 10.1002/ar.1092010309. [DOI] [PubMed] [Google Scholar]
- Murray R. E., Gibson J. E. A comparative study of paraquat intoxication in rats, guinea pigs and monkeys. Exp Mol Pathol. 1972 Dec;17(3):317–325. doi: 10.1016/0014-4800(72)90044-5. [DOI] [PubMed] [Google Scholar]
- Payan H., Monges G., Jouve M. P., Saux M. A., Pellegrin E., Garbe L. Intoxication par le paraquat. Etude ultrastructurale des lésions pulmonaires à propos d'une observation. Arch Anat Cytol Pathol. 1982;30(1):33–38. [PubMed] [Google Scholar]
- Rebello G., Mason J. K. Pulmonary histological appearances in fatal paraquat poisoning. Histopathology. 1978 Jan;2(1):53–66. doi: 10.1111/j.1365-2559.1978.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Reiser K. M., Last J. A. Pulmonary fibrosis in experimental acute respiratory disease. Am Rev Respir Dis. 1981 Jan;123(1):58–63. doi: 10.1164/arrd.1981.123.1.58. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Church R. L., Rohrbach D. H., Shupp D. E., Abe S., Hewitt A. T., Murray J. C., Martin G. R. Localization of the human fibronectin (FN) gene on chromosome 8 by a specific enzyme immunoassay. Biochem Genet. 1981 Jun;19(5-6):551–566. doi: 10.1007/BF00484626. [DOI] [PubMed] [Google Scholar]
- Rentería V. G., Ferrans V. J. Intracellular collagen fibrils in cardiac valves of patients with the Hurler syndrome. Lab Invest. 1976 Mar;34(3):263–272. [PubMed] [Google Scholar]
- Ross R., Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969 Feb;40(2):366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger C. I., Rennard S. I., Bitterman P. B., Fukuda Y., Ferrans V. J., Crystal R. G. Paraquat-induced pulmonary fibrosis. Role of the alveolitis in modulating the development of fibrosis. Am Rev Respir Dis. 1984 Jan;129(1):168–173. doi: 10.1164/arrd.1984.129.1.168. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J. J., Wycoff D., Zavala D. C., Richerson H. B. Paraquat lung injury in rabbits. Br J Ind Med. 1978 Aug;35(3):245–257. doi: 10.1136/oem.35.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976 Jun;57(6):1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. T., Fletcher W. A. Pulmonary epithelial-mesenchymal interactions: beyond organogenesis. Hum Pathol. 1979 May;10(3):248–250. doi: 10.1016/s0046-8177(79)80020-9. [DOI] [PubMed] [Google Scholar]
- Smith P., Heath D., Kay J. M. The pathogenesis and structure of paraquat-induced pulmonary fibrosis in rats. J Pathol. 1974 Oct;114(2):57–67. doi: 10.1002/path.1711140202. [DOI] [PubMed] [Google Scholar]
- Smith P., Heath D. Paraquat lung: a reappraisal. Thorax. 1974 Nov;29(6):643–653. doi: 10.1136/thx.29.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P., Heath D. The pathology of the lung in paraquat poisoning. J Clin Pathol Suppl (R Coll Pathol) 1975;9:81–93. [PMC free article] [PubMed] [Google Scholar]
- Stephens R. J., Freeman G., Evans M. J. Ultrastructural changes in connective tissue in lungs of rats exposed to NO 2 . Arch Intern Med. 1971 May;127(5):873–883. [PubMed] [Google Scholar]
- Toner P. G., Vetters J. M., Spilg W. G., Harland W. A. Fine structure of the lung lesion in a case of paraquat poisoning. J Pathol. 1970 Nov;102(3):182–185. doi: 10.1002/path.1711020311. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L. Mesenchymal cell polarity and morphogenesis of chick cartilage. Dev Biol. 1977 Sep;59(2):153–163. doi: 10.1016/0012-1606(77)90250-0. [DOI] [PubMed] [Google Scholar]
- Vijeyaratnam G. S., Corrin B. Experimental paraquat poisoning: a histological and electron-optical study of the changes in the lung. J Pathol. 1971 Feb;103(2):123–129. doi: 10.1002/path.1711030207. [DOI] [PubMed] [Google Scholar]
- Vracko R. Significance of basal lamina for regeneration of injured lung. Virchows Arch A Pathol Pathol Anat. 1972;355(3):264–274. doi: 10.1007/BF00551062. [DOI] [PubMed] [Google Scholar]