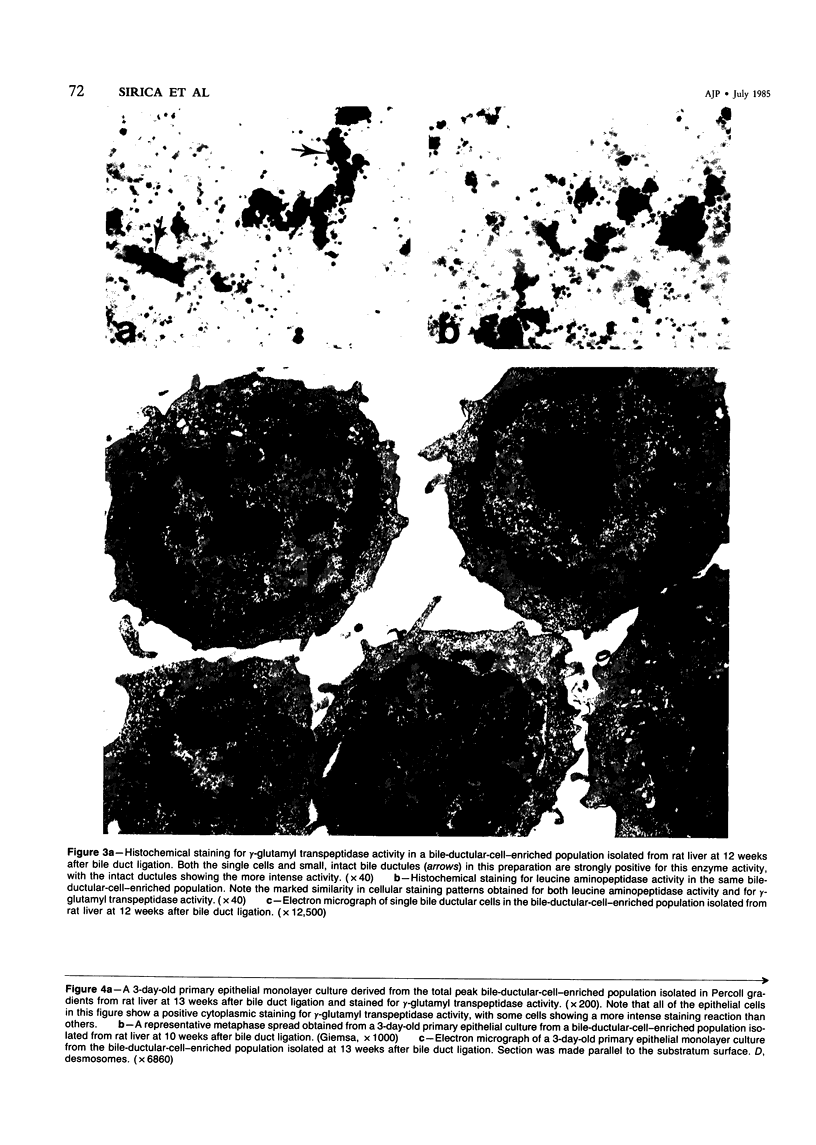
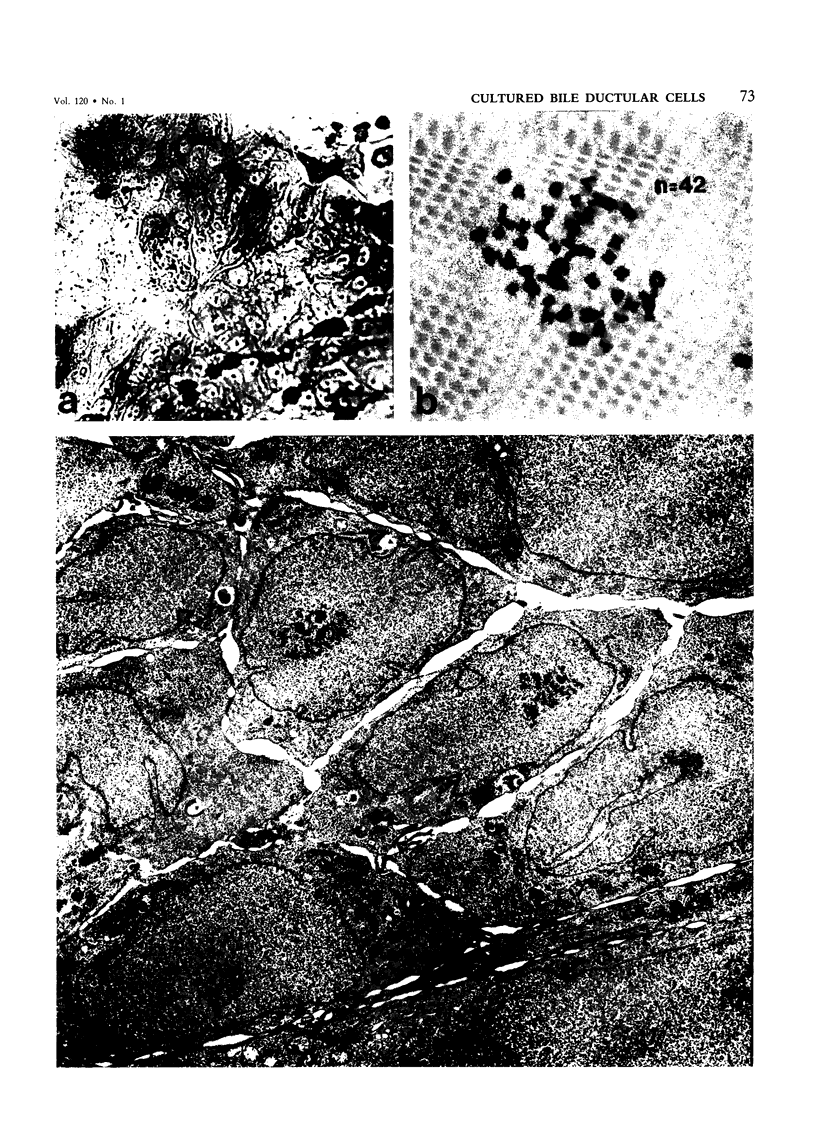
Abstract
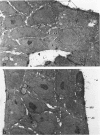
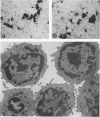
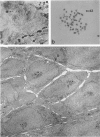
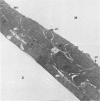
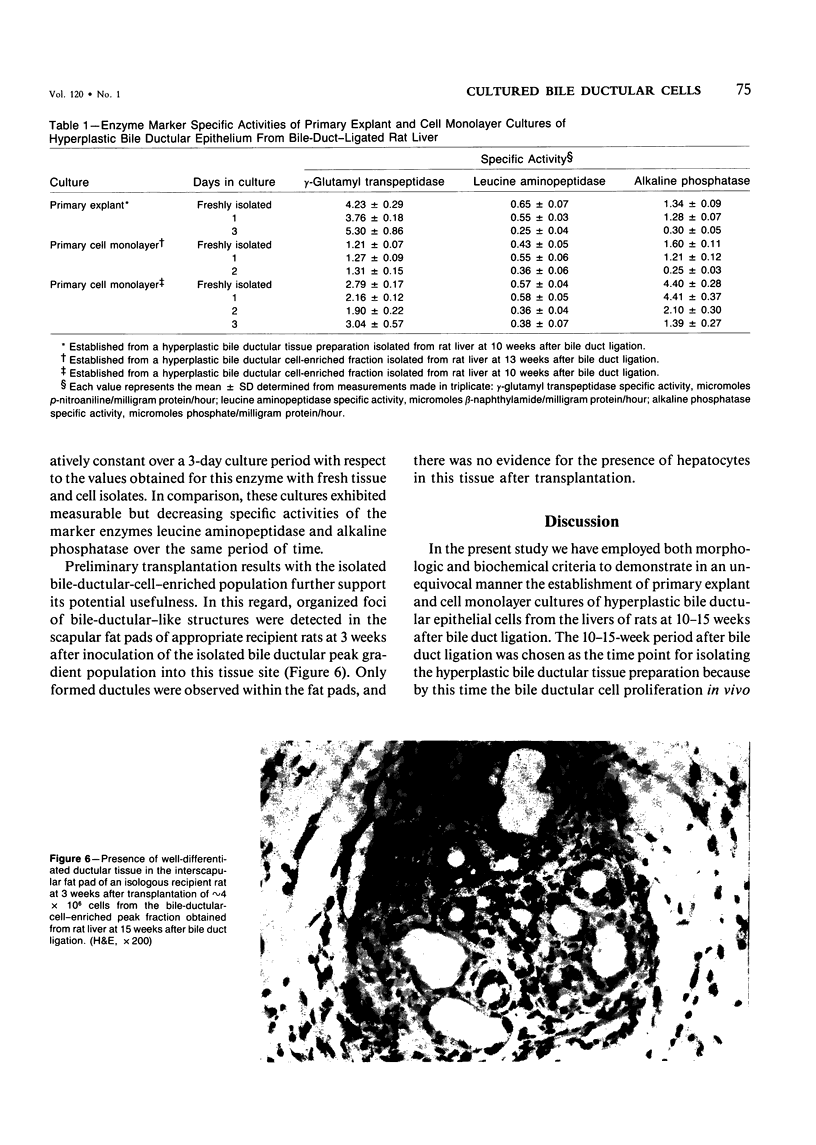
The establishment of novel bile ductular cell cultures was accomplished with the use of explants of a hyperplastic bile ductular tissue preparation obtained from rat livers at 10 to 15 weeks after bile duct ligation or a bile ductular cell fraction isolated from this tissue preparation by a procedure involving Percoll density gradient centrifugation. Observations made on these primary explant and monolayer bile ductular cell cultures were limited to the first 3 days of culture where the morphologic features of the bile ductular epithelium remained fairly well preserved, while fibroblast contamination was found to be very low. These cultured cells also retained over this period a high specific activity for the bile ductular cell marker enzyme gamma-glutamyl transpeptidase, as well as possessed measurable but decreasing specific activities for leucine aminopeptidase and alkaline phosphatase. Karyotypic analysis of the cultured monolayer cells further showed them to be diploid. In addition, preliminary transplantation studies demonstrated the presence of well-differentiated bile ductular-like structures following inoculation of the freshly isolated bile ductular cell fraction into the interscapular fat pads of recipient rats.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Murphy P., Richardson L. C. Procedure for embedding in situ selected cells cultured in vitro. J Cell Biol. 1967 Oct;35(1):279–283. doi: 10.1083/jcb.35.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. T., Gibson J. B. A histochemical study of the bile ducts in long-term biliary obstruction in the rat. J Pathol. 1971 Mar;103(3):163–175. doi: 10.1002/path.1711030305. [DOI] [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Grant A. G., Billing B. H. The isolation and characterization of a bile ductule cell population from normal and bile-duct ligated rat livers. Br J Exp Pathol. 1977 Jun;58(3):301–310. [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in rat liver cultures: their identification and isolation. Mol Cell Biochem. 1983;53-54(1-2):23–33. doi: 10.1007/BF00225244. [DOI] [PubMed] [Google Scholar]
- Hayner N. T., Braun L., Yaswen P., Brooks M., Fausto N. Isozyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):332–338. [PubMed] [Google Scholar]
- Huberman E., Montesano R., Drevon C., Kuroki T., St Vincent L., Pugh T. D., Goldfarb S. gamma-Glutamyl transpeptidase and malignant transformation of cultured liver cells. Cancer Res. 1979 Jan;39(1):269–272. [PubMed] [Google Scholar]
- Jirtle R. L., Biles C., Michalopoulos G. Morphologic and histochemical analysis of hepatocytes transplanted into syngeneic hosts. Am J Pathol. 1980 Oct;101(1):115–126. [PMC free article] [PubMed] [Google Scholar]
- Jirtle R. L., Michalopoulos G., McLain J. R., Crowley J. Transplantation system for determining the clonogenic survival of parenchymal hepatocytes exposed to ionizing radiation. Cancer Res. 1981 Sep;41(9 Pt 1):3512–3518. [PubMed] [Google Scholar]
- Kaplan M. M., Kanel G. C., Singer J. A. Enzyme changes and morphometric analysis of bile ducts in experimental bile duct obstruction. Clin Chim Acta. 1979 Dec 3;99(2):113–119. doi: 10.1016/0009-8981(79)90033-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledda G. M., Sells M. A., Yokoyama S., Lombardi B. Metabolic properties of isolated rat liver cell preparations enriched in epithelial cells other than hepatocytes. Int J Cancer. 1983 Feb 15;31(2):231–237. doi: 10.1002/ijc.2910310217. [DOI] [PubMed] [Google Scholar]
- Leonard T. B., Neptun D. A., Popp J. A. Serum gamma glutamyl transferase as a specific indicator of bile duct lesions in the rat liver. Am J Pathol. 1984 Aug;116(2):262–269. [PMC free article] [PubMed] [Google Scholar]
- NACHLAS M. M., CRAWFORD D. T., SELIGMAN A. M. The histochemical demonstration of leucine aminopeptidase. J Histochem Cytochem. 1957 May;5(3):264–278. doi: 10.1177/5.3.264. [DOI] [PubMed] [Google Scholar]
- Oda M., Yousef I. M., Phillips M. J. Isolation of bile ducts from rat liver: technique and preliminary ultrastructural characterization. Exp Mol Pathol. 1975 Oct;23(2):214–219. doi: 10.1016/0014-4800(75)90019-2. [DOI] [PubMed] [Google Scholar]
- RUBIN E. THE ORIGIN AND FATE OF PROLIFERATED BILE DUCTULAR CELLS. Exp Mol Pathol. 1964 Jun;86:279–286. doi: 10.1016/0014-4800(64)90059-0. [DOI] [PubMed] [Google Scholar]
- Richards W. L., Tsukada Y., Potter V. R. gamma-Glutamyl transpeptidase and alpha-fetoprotein expression during alpha-naphthylisothiocyanate-induced hepatotoxicity in rats. Cancer Res. 1982 Dec;42(12):5133–5138. [PubMed] [Google Scholar]
- STEINER J. W., CARRUTHERS J. S., KALIFAT S. R. The ductular cell reaction of rat liver in extrahepatic cholestasis. I. Proliferated biliary epithelial cells. Exp Mol Pathol. 1962 Apr;1:162–185. doi: 10.1016/0014-4800(62)90019-9. [DOI] [PubMed] [Google Scholar]
- San R. H., Shimada T., Maslansky C. J., Kreiser D. M., Laspia M. F., Rice J. M., Williams G. M. Growth characteristics and enzyme activities in a survey of transformation markers in adult rat liver epithelial-like cell cultures. Cancer Res. 1979 Nov;39(11):4441–4448. [PubMed] [Google Scholar]
- Sell S., Leffert H. L. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982 Jan-Feb;2(1):77–86. doi: 10.1002/hep.1840020113. [DOI] [PubMed] [Google Scholar]
- Sells M. A., Katyal S. L., Shinozuka H., Estes L. W., Sell S., Lombardi B. Isolation of oval cells and transitional cells from the livers of rats fed the carcinogen DL-ethionine. J Natl Cancer Inst. 1981 Feb;66(2):355–362. [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R. M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978 Apr;38(4):1092–1098. [PubMed] [Google Scholar]
- Sirica A. E., Cihla H. P. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984 Aug;44(8):3454–3466. [PubMed] [Google Scholar]
- Sirica A. E., Goldblatt P. J., McKelvy J. F. Isolation and partial characterization of a bile canalicular plasma membrane fraction from normal and regenerating rat liver. J Biol Chem. 1975 Aug 25;250(16):6464–6468. [PubMed] [Google Scholar]
- Sirica A. E., Pitot H. C. Drug metabolism and effects of carcinogens in cultured hepatic cells. Pharmacol Rev. 1979 Sep;31(3):205–228. [PubMed] [Google Scholar]
- Sirica A. E., Richards W., Tsukada Y., Sattler C. A., Pitot H. C. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Smith J. D., Nelson K. G., Grisham J. W. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res. 1984 Sep;154(1):38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- Wootton A. M., Neale G., Moss D. W. Some properties of alkaline-phosphatases in parenchymal and biliary tract cells separated from rat liver. Clin Chim Acta. 1975 Jun 2;61(2):183–190. doi: 10.1016/0009-8981(75)90313-7. [DOI] [PubMed] [Google Scholar]
- Wu P. C., Ma L., Gibson J. B., Hirai H., Tsukada Y. Serum alpha-fetoprotein in rats after ligation of the common bile duct: relation to ductular cell (oval cell) proliferation. J Pathol. 1981 Jan;133(1):61–74. doi: 10.1002/path.1711330107. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Hayner N. T., Fausto N. Isolation of oval cells by centrifugal elutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1984 Jan;44(1):324–331. [PubMed] [Google Scholar]
- Yoshimura H., Harris R., Yokoyama S., Takahashi S., Sells M. A., Pan S. F., Lombardi B. Anaplastic carcinomas in nude mice and in original donor strain rats inoculated with cultured oval cells. Am J Pathol. 1983 Mar;110(3):322–332. [PMC free article] [PubMed] [Google Scholar]