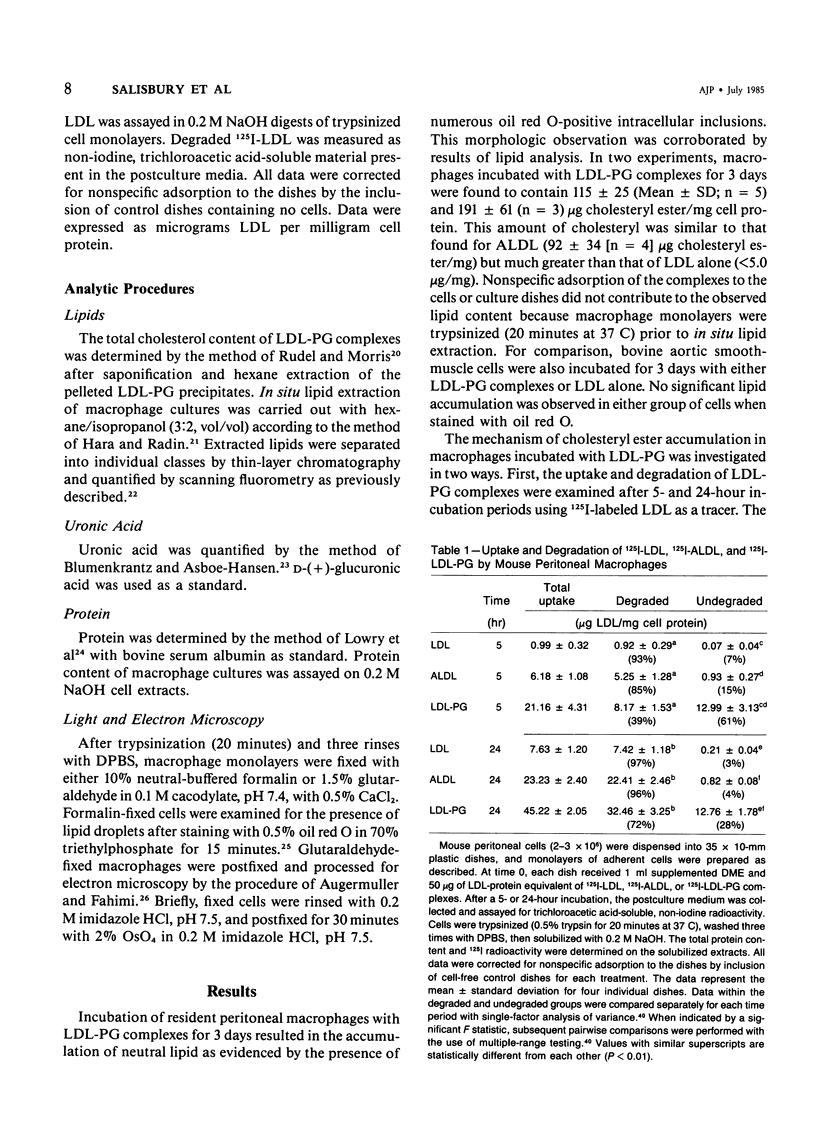
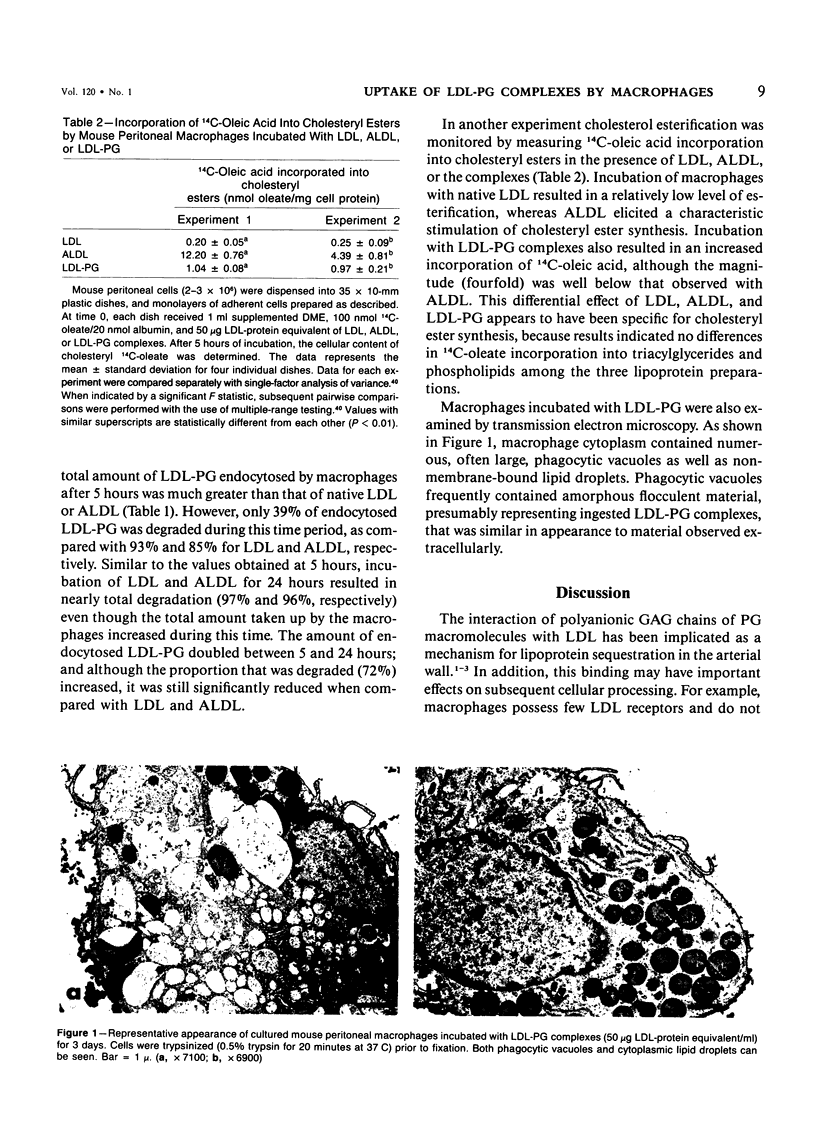
Abstract
The interaction of arterial proteoglycans (PGs) and low-density lipoproteins (LDLs) has been postulated to be an important factor in extracellular cholesterol accumulation in the arterial wall. In the present study, insoluble complexes of LDL and PG (LDL-PG) were prepared and their effects on cholesteryl ester accumulation in mouse peritoneal macrophages was tested. The cholesteryl ester content of macrophages incubated with LDL-PG for 3 days was greater than 20 times that observed in cells incubated with LDL alone. The uptake of 125I-LDL by macrophages was markedly stimulated if LDL was incorporated into a complex with PG. However, in contrast to either LDL or acetylated LDL (ALDL), the extent of subsequent degradation of LDL-PG by the cells was reduced. The uptake and degradation of LDL-PG complexes stimulated macrophage incorporation of 14C-oleic acid into cholesteryl oleate 4- to 5-fold over LDL alone; however, esterification was significantly less than that observed with ALDL, even though more LDL-PG was degraded. Ultrastructurally, macrophages incubated with LDL-PG complexes contained lipid droplets as well as numerous phagocytic vacuoles often containing material similar in appearance to insoluble complexes. These results demonstrate that components of the extracellular matrix, such as PG, can modify the catabolism of LDL by scavenger cells. This phenomenon may be potentially important with respect to foam-cell genesis from macrophages in the arterial wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angermüller S., Fahimi H. D. Imidazole-buffered osmium tetroxide: an excellent stain for visualization of lipids in transmission electron microscopy. Histochem J. 1982 Sep;14(5):823–835. doi: 10.1007/BF01033631. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Inhibition of leucocytic lysosomal enzymes by glycosaminoglycans in vitro. Biochem J. 1975 Oct;152(1):57–64. doi: 10.1042/bj1520057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Physicochemical characteristics of the glycosaminoglycan-lysosomal enzyme interaction in vitro. A model of control of leucocytic lysosomal activity. Biochem J. 1976 Nov 15;160(2):129–136. doi: 10.1042/bj1600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Goldstein J. L. Degradation of low density lipoprotein . dextran sulfate complexes associated with deposition of cholesteryl esters in mouse macrophages. J Biol Chem. 1979 Aug 10;254(15):7141–7146. [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Srinivasan S. R., Vijayagopal P., Dalferes E. R., Jr, Sharma C. Recent advances in molecular pathology. Carbohydrate-protein macromolecules and arterial wall integrity--a role in atherogenesis. Exp Mol Pathol. 1984 Oct;41(2):267–287. doi: 10.1016/0014-4800(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Camejo G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: its possible role in atherogenesis. Adv Lipid Res. 1982;19:1–53. doi: 10.1016/b978-0-12-024919-0.50007-2. [DOI] [PubMed] [Google Scholar]
- Clevidence B. A., Morton R. E., West G., Dusek D. M., Hoff H. F. Cholesterol esterification in macrophages. Stimulation by lipoproteins containing apo B isolated from human aortas. Arteriosclerosis. 1984 May-Jun;4(3):196–207. doi: 10.1161/01.atv.4.3.196. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., Hajjar D. P., Minick C. R. Enhancement of cholesterol and cholesteryl ester accumulation in re-endothelialized aorta. Am J Pathol. 1980 Apr;99(1):81–104. [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., Mated N., Shio H., Minick C. R., Fowler S. D. Lipoprotein-heparin-fibronectin-denatured collagen complexes enhance cholesteryl ester accumulation in macrophages. J Cell Biol. 1984 Oct;99(4 Pt 1):1266–1274. doi: 10.1083/jcb.99.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brown M. S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Radin N. S. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978 Oct 1;90(1):420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Heideman C. L., Gaubatz J. W., Scott D. W., Gotto A. M., Jr Detergent extraction of tightly-bound apoB from extracts of normal aortic intima and plaques. Exp Mol Pathol. 1978 Jun;28(3):290–300. doi: 10.1016/0014-4800(78)90003-5. [DOI] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Intralysosomal accumulation of polyanions. II. Polyanion internalization and its influence on lysosomal pH and membrane fluidity. J Cell Biol. 1982 Jun;93(3):875–882. doi: 10.1083/jcb.93.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Salisbury B. G., Wagner W. D. Isolation and preliminary characterization of proteoglycans dissociatively extracted from human aorta. J Biol Chem. 1981 Aug 10;256(15):8050–8057. [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S. R., Dolan P., Radhakrishnamurthy B., Berenson G. S. Isolation of lipoprotein-acid mucopolysaccharide complexes from fatty streaks of human aortas. Atherosclerosis. 1972 Jul-Aug;16(1):95–104. doi: 10.1016/0021-9150(72)90012-3. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. R., Radhakrishnamurthy B., Pargaonkar P. S., Berenson G. S., Dolan P. Lipoprotein-acid mucopolysaccharide complexes of human atherosclerotic lesions. Biochim Biophys Acta. 1975 Apr 18;388(1):58–70. doi: 10.1016/0005-2760(75)90062-4. [DOI] [PubMed] [Google Scholar]
- Traber M. G., Kayden H. J. Low density lipoprotein receptor activity in human monocyte-derived macrophages and its relation to atheromatous lesions. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5466–5470. doi: 10.1073/pnas.77.9.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayagopal P., Radhakrishnamurthy B., Srinivasan S. R., Berenson G. S. Studies of biologic properties of proteoglycans from bovine aorta. Lab Invest. 1980 Feb;42(2):190–196. [PubMed] [Google Scholar]
- Vijayagopal P., Srinivasan S. R., Radhakrishnamurthy B., Berenson G. S. Hemostatic properties and serum lipoprotein binding of a heparan sulfate proteoglycan from bovine aorta. Biochim Biophys Acta. 1983 Jul 5;758(1):70–83. doi: 10.1016/0304-4165(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Wagner W. D., Salisbury B. G. Aortic total glycosaminoglycan and dermatan sulfate changes in atherosclerotic rhesus monkeys. Lab Invest. 1978 Oct;39(4):322–328. [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Cholesterol metabolism in the macrophage. 3. Ingestion and intracellular fate of cholesterol and cholesterol esters. J Exp Med. 1972 Jan;135(1):21–44. doi: 10.1084/jem.135.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N. Vessel proteoglycans and thrombogenesis. Prog Hemost Thromb. 1980;5:1–39. [PubMed] [Google Scholar]



