Abstract
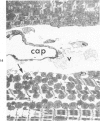
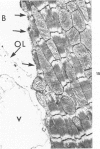
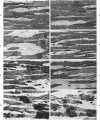
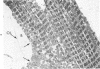
It has not previously been possible to study the in vitro effects of reperfusion on severely injured isolated perfused hearts because of the development of the no-reflow phenomenon, concomitant with the onset of irreversible myocardial cell injury. A new model of ischemic injury which utilizes an intraventricular balloon to allow uniform reperfusion of irreversibly damaged hearts is described. The effects of reperfusion were studied in Langendorff perfused rat hearts after no-flow ischemia for 60 and 150 minutes at 37 C. Uniform reflow was facilitated by maintaining the left ventricle at an isovolumic diastolic volume with a balloon during ischemia and removal of the balloon prior to reflow. Reperfusion was with 1) anoxic media, 2) oxygenated media, 3) oxygenated media in the presence of the mitochondrial inhibitor Amytal, or 4) an initial anoxic reperfusion followed by oxygenated media. Injury was monitored by the assay of released creatine kinase (CK) and myoglobin (Myo), by light-microscopic estimates of the percent of cells containing contraction bands, and by ultrastructural changes. CK and Myo were released with anoxic reperfusion, but larger releases occurred with oxygenated reperfusion. Amytal inhibited the oxygen but not the nitrogen component of release. Contraction bands occurred following oxygenated, but not anoxic, reperfusions and were inhibited by Amytal. Following an initial anoxic reperfusion, oxygen caused additional CK and Myo release and produced an increase in the percent of cells with contraction bands, compared with that with oxygen alone. The response of cells to injury was heterogeneous, and the hearts contained cells with a spectrum of ultrastructural changes. Anoxic reperfusion was associated with cellular swelling and oxygenated reperfusion with contraction band necrosis.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apstein C. S., Mueller M., Hood W. B., Jr Ventricular contracture and compliance changes with global ischemia and reperfusion, and their effect on coronary resistance in the rat. Circ Res. 1977 Aug;41(2):206–217. doi: 10.1161/01.res.41.2.206. [DOI] [PubMed] [Google Scholar]
- Ashraf M., White F., Bloor C. M. Ultrastructural influence of reperfusing dog myocardium with calcium-free blood after coronary artery occlusion. Am J Pathol. 1978 Feb;90(2):423–434. [PMC free article] [PubMed] [Google Scholar]
- Buja L. M., Willerson J. T. Abnormalities of volume regulation and membrane integrity in myocardial tissue slices after early ischemic injury in the dog: effects of mannitol, polyethylene glycol, and propranolol. Am J Pathol. 1981 Apr;103(1):79–95. [PMC free article] [PubMed] [Google Scholar]
- Crozatier B., Ashraf M., Franklin D., Ross J., Jr Sarcomere length in experimental myocardial infarction: evidence for sarcomere overstretch in dyskinetic ventricular regions. J Mol Cell Cardiol. 1977 Oct;9(10):785–797. doi: 10.1016/s0022-2828(77)80056-4. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Powell W. J., Jr Quantitative correlation between cell swelling and necrosis in myocardial ischemia in dogs. Circ Res. 1980 Nov;47(5):653–665. doi: 10.1161/01.res.47.5.653. [DOI] [PubMed] [Google Scholar]
- Ganote C. E. Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol. 1983 Feb;15(2):67–73. doi: 10.1016/0022-2828(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Jennings R. B., Hill M. L., Grochowski E. Experimental myocardial ischemic injury. II. Effect of in vivo ischemia on dog heart slice function in vitro. J Mol Cell Cardiol. 1976 Mar;8(3):189–204. doi: 10.1016/0022-2828(76)90036-5. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Kaltenbach J. P. Oxygen-induced enzyme release: early events and a proposed mechanism. J Mol Cell Cardiol. 1979 Apr;11(4):389–406. doi: 10.1016/0022-2828(79)90425-5. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., McGarr J., Liu S. Y., Kaltenbach J. P. Oxygen-induced enzyme release. Assessment of mitochondrial function in anoxic myocardial injury and effects of the mitochondrial uncoupling agent 2,4-dinitrophenol (DNP). J Mol Cell Cardiol. 1980 Apr;12(4):387–408. doi: 10.1016/0022-2828(80)90049-8. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Worstell J., Iannotti J. P., Kaltenbach J. P. Cellular swelling and irreversible myocardial injury. Effects of polyethylene glycol and mannitol in perfused rat hearts. Am J Pathol. 1977 Jul;88(1):95–118. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Worstell J., Kaltenbach J. P. Oxygen-induced enzyme release after irreversible myocardial injury. Effects of cyanide in perfused rat hearts. Am J Pathol. 1976 Aug;84(2):327–350. [PMC free article] [PubMed] [Google Scholar]
- Grinwald P. M. Calcium uptake during post-ischemic reperfusion in the isolated rat heart: influence of extracellular sodium. J Mol Cell Cardiol. 1982 Jun;14(6):359–365. doi: 10.1016/0022-2828(82)90251-6. [DOI] [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Nayler W. G., Slade A., Border D. Ultrastructural damage associated with reoxygenation of the anoxic myocardium. J Mol Cell Cardiol. 1975 May;7(5):315–324. doi: 10.1016/0022-2828(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Hearse D. J. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977 Aug;9(8):605–616. doi: 10.1016/s0022-2828(77)80357-x. [DOI] [PubMed] [Google Scholar]
- Humphrey S. M., Gavin J. B., Herdson P. B. The relationship of ischemic contracture of vascular reperfusion in the isolated rat heart. J Mol Cell Cardiol. 1980 Dec;12(12):1397–1406. doi: 10.1016/0022-2828(80)90124-8. [DOI] [PubMed] [Google Scholar]
- Humphrey S. M., Thomson R. W., Gavin J. B. The effect of an isovolumic left ventricle on the coronary vascular competence during reflow after global ischemia in the rat heart. Circ Res. 1981 Sep;49(3):784–791. doi: 10.1161/01.res.49.3.784. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Tiozzo R., Lugli G., Lehninger A. L., Carafoli E. Aspects of energy-linked calcium accumulation by rat heart mitochondria. J Biol Chem. 1975 Oct 10;250(19):7863–7870. [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E., Reimer K. A. Ischemic tissue injury. Am J Pathol. 1975 Oct;81(1):179–198. [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. Lethal myocardial ischemic injury. Am J Pathol. 1981 Feb;102(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Koomen J. M., Schevers J. A., Noordhoek J. Myocardial recovery from global ischemia and reperfusion: effects of pre- and/or post-ischemic perfusion with low-Ca2+. J Mol Cell Cardiol. 1983 Jun;15(6):383–392. doi: 10.1016/0022-2828(83)90322-x. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Sodium-calcium exchange in the heart. Annu Rev Physiol. 1982;44:435–449. doi: 10.1146/annurev.ph.44.030182.002251. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Poole-Wilson P. A., Williams A. Hypoxia and calcium. J Mol Cell Cardiol. 1979 Jul;11(7):683–706. doi: 10.1016/0022-2828(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Nayler W. G. The role of calcium in the ischemic myocardium. Am J Pathol. 1981 Feb;102(2):262–270. [PMC free article] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Harding D. P., Bourdillon P. D., Tones M. A. Calcium out of control. J Mol Cell Cardiol. 1984 Feb;16(2):175–187. doi: 10.1016/s0022-2828(84)80706-3. [DOI] [PubMed] [Google Scholar]
- Shine K. I., Douglas A. M. Low calcium reperfusion of ischemic myocardium. J Mol Cell Cardiol. 1983 Apr;15(4):251–260. doi: 10.1016/0022-2828(83)90280-8. [DOI] [PubMed] [Google Scholar]
- Tranum-Jensen J., Janse M. J., Fiolet W. T., Krieger W. J., D'Alnoncourt C. N., Durrer D. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ Res. 1981 Aug;49(2):364–381. doi: 10.1161/01.res.49.2.364. [DOI] [PubMed] [Google Scholar]