Abstract
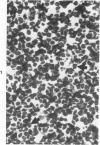
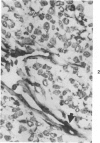
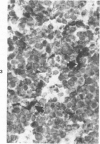
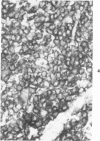
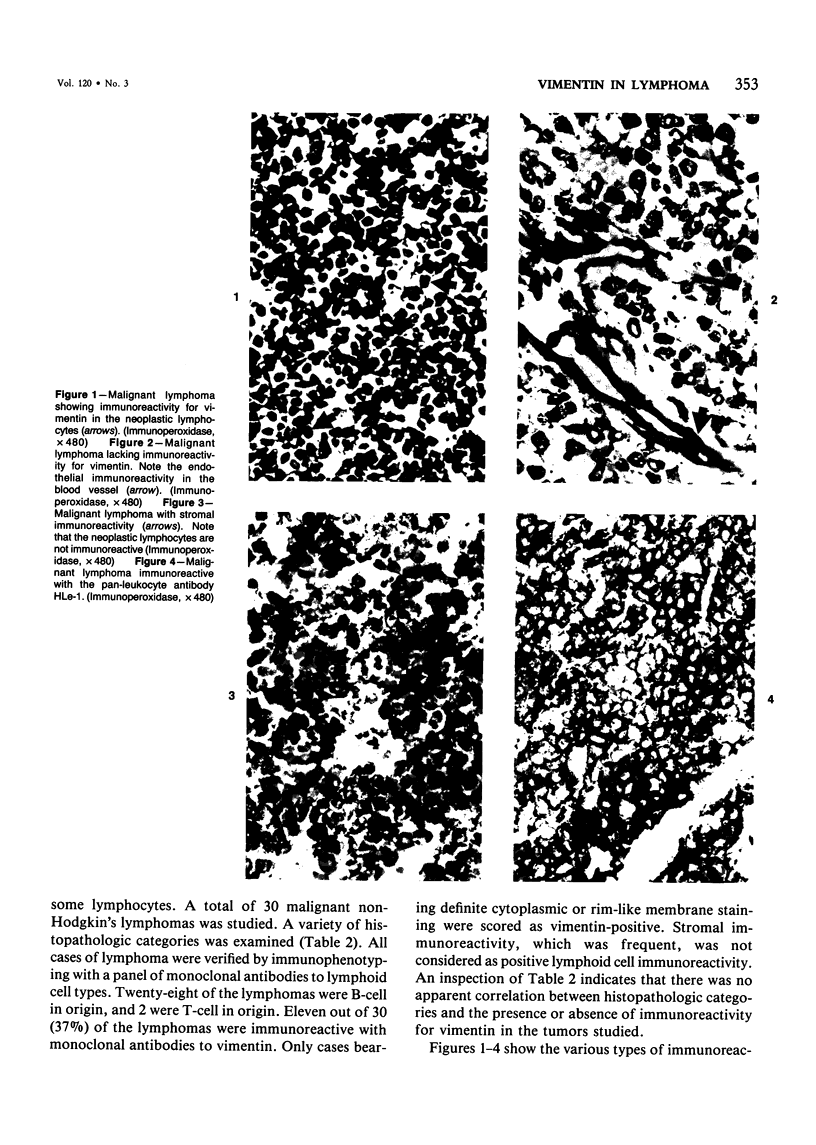
A series of human non-Hodgkin's lymphomas was examined for immunoreactivity with monoclonal antibodies to the intermediate filament protein vimentin with the use of an avidin-biotin immunoperoxidase method. The lymphoid cell nature of each tumor was established with the use of a panel of monoclonal antibodies to lymphoid cell differentiation antigens. There were 28 B-cell and 2 T-cell lymphomas in the series; of the 30 tumors, 11 (37%) were immunoreactive for vimentin. There was no correlation between vimentin immunoreactivity and the histopathologic type of lymphoma. In some tumors, there was nonspecific stromal immunoreactivity for vimentin, but the neoplastic lymphocytes were not immunoreactive. The selective expression of vimentin in non-Hodgkin's lymphomas may be due to masking of the appropriate epitopes or to selective expression of the vimentin gene in certain tumors. On the basis of these results, monoclonal antibodies to vimentin appear to be of limited usefulness in establishing the diagnosis of non-Hodgkin's lymphoma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. D., Hulsebosch H. J., Krieg S. R., Bakker P. M., Cormane R. H. Immunocompetent cells in psoriasis. In situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275(3):181–189. doi: 10.1007/BF00510050. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno R. A comparison of two immunoperoxidase staining methods based on the avidin-biotin interaction. Diagn Immunol. 1984;2(3):161–166. [PubMed] [Google Scholar]
- Giorno R. Immunohistochemical analysis of the distribution of vimentin in human peripheral lymphoid tissues. Anat Rec. 1985 Jan;211(1):43–47. doi: 10.1002/ar.1092110108. [DOI] [PubMed] [Google Scholar]
- Giorno R., Kohler P. F. Immunohistological localization of human lymphocyte subsets. Diagn Immunol. 1983;1(1):17–26. [PubMed] [Google Scholar]
- Giorno R. Technical considerations in immunohistology of lymphoid cell membrane antigens. Surv Synth Pathol Res. 1984;3(2):165–188. doi: 10.1159/000156924. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Schaumburg H. H., Norton W. T. Isolation and characterization of glial filaments from human brain. J Cell Biol. 1978 Aug;78(2):426–440. doi: 10.1083/jcb.78.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Ambrose W. W., James C. J., Mandelkorn J., Yates P. E., Gall S. A., Bossen E. H., Fay J. W., Laszlo J., Moore J. O. Facilitated light microscopic cytochemical diagnosis of acute myelogenous leukemia. Cancer Res. 1979 May;39(5):1635–1639. [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Kahn H. J., Yeger H., Baumal R., Thom H., Phillips J. M. Categorization of pediatric neoplasms by immunostaining with antiprekeratin and antivimentin antisera. Cancer. 1983 Feb 15;51(4):645–653. doi: 10.1002/1097-0142(19830215)51:4<645::aid-cncr2820510417>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human b-cell differentiation. I. Analysis of immunoglobulin heavy chain switching using monoclonal anti-immunoglobulin M, G, and A antibodies and pokeweed mitogen-induced plasma cell differentiation. J Exp Med. 1982 Mar 1;155(3):839–851. doi: 10.1084/jem.155.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem R. K., Yen S. H., Salomon G. D., Shelanski M. L. Intermediate filaments in nervous tissues. J Cell Biol. 1978 Dec;79(3):637–645. doi: 10.1083/jcb.79.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller M., Glistrup O. V., Olsen W. Contrast enhancement of the brownish horseradish peroxidase-activated 3,3'-diaminobenzidine tetrahydrochloride reaction product in black and white photomicrography by the use of interference filters. J Histochem Cytochem. 1984 Jan;32(1):37–42. doi: 10.1177/32.1.6690599. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pizzolo G., Sloane J., Beverley P., Thomas J. A., Bradstock K. F., Mattingly S., Janossy G. Differential diagnosis of malignant lymphoma and nonlymphoid tumors using monoclonal anti-leucocyte antibody. Cancer. 1980 Dec 15;46(12):2640–2647. doi: 10.1002/1097-0142(19801215)46:12<2640::aid-cncr2820461218>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Kant A., Jap P., Herman C., Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology. Lab Invest. 1983 Sep;49(3):353–361. [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]