Abstract
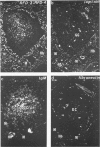
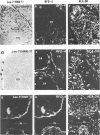
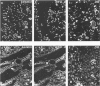
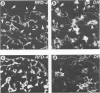
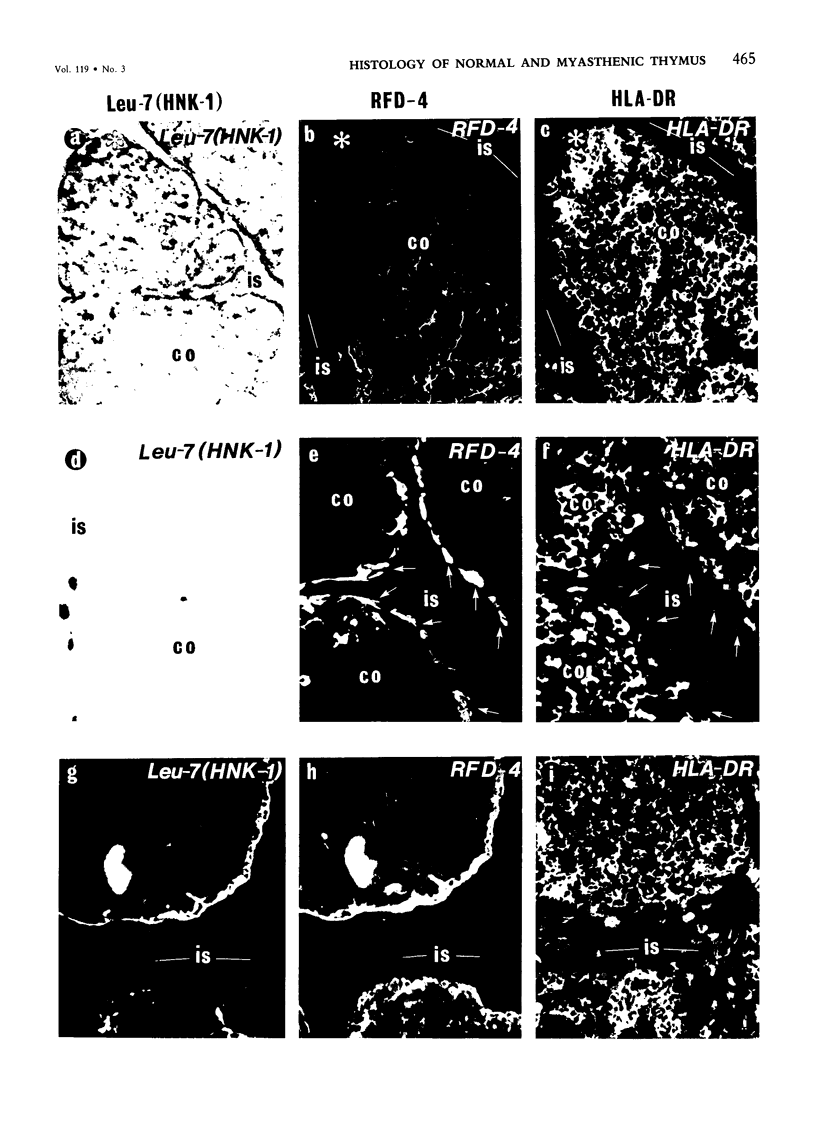
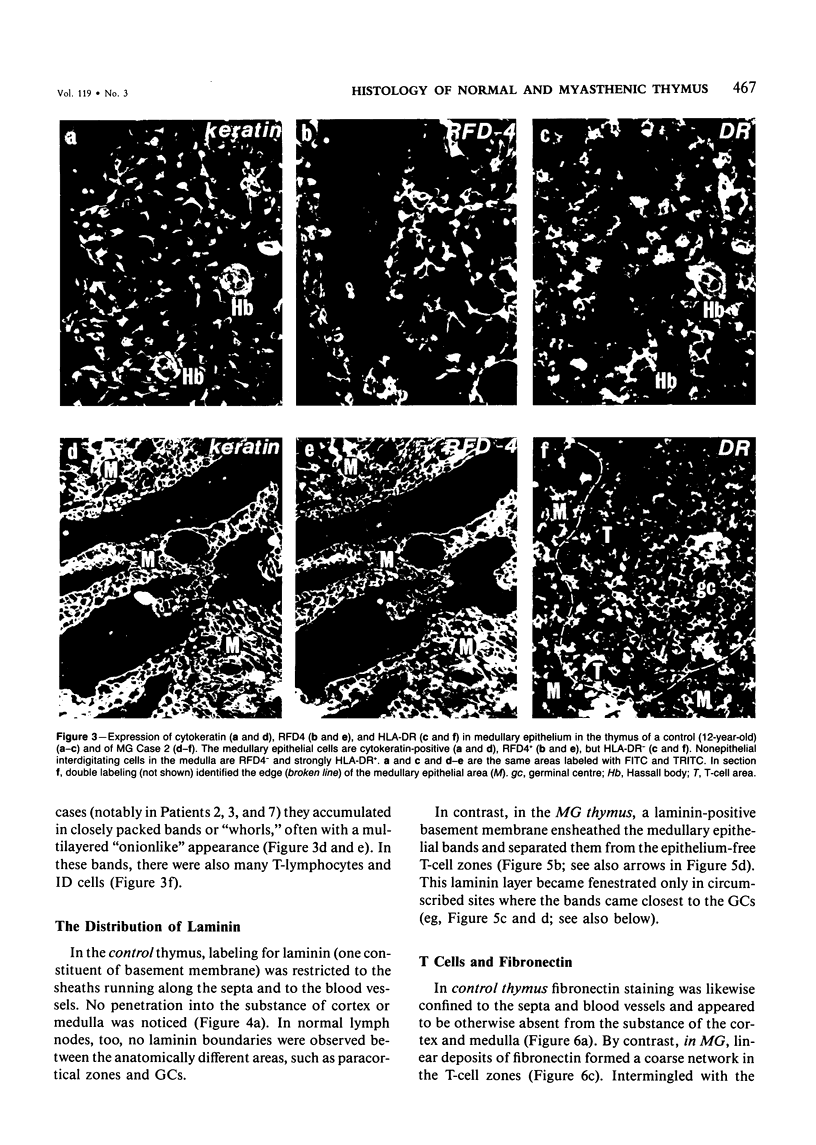
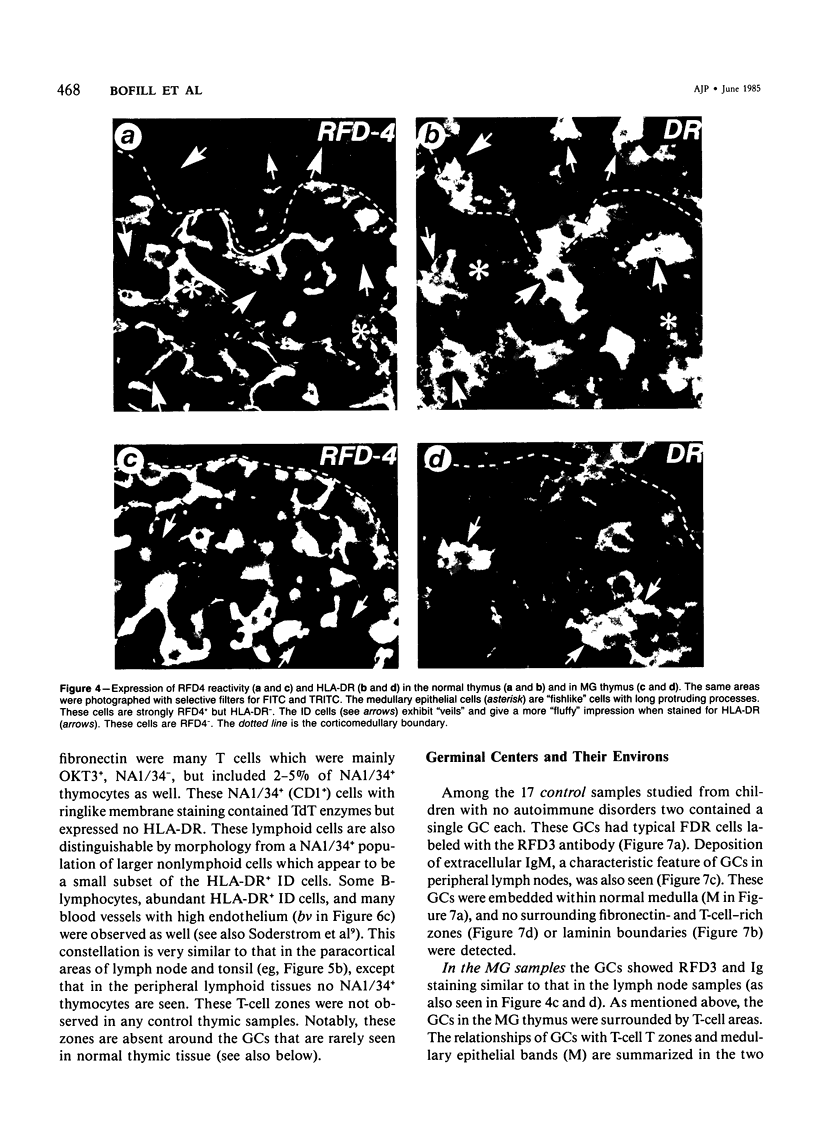
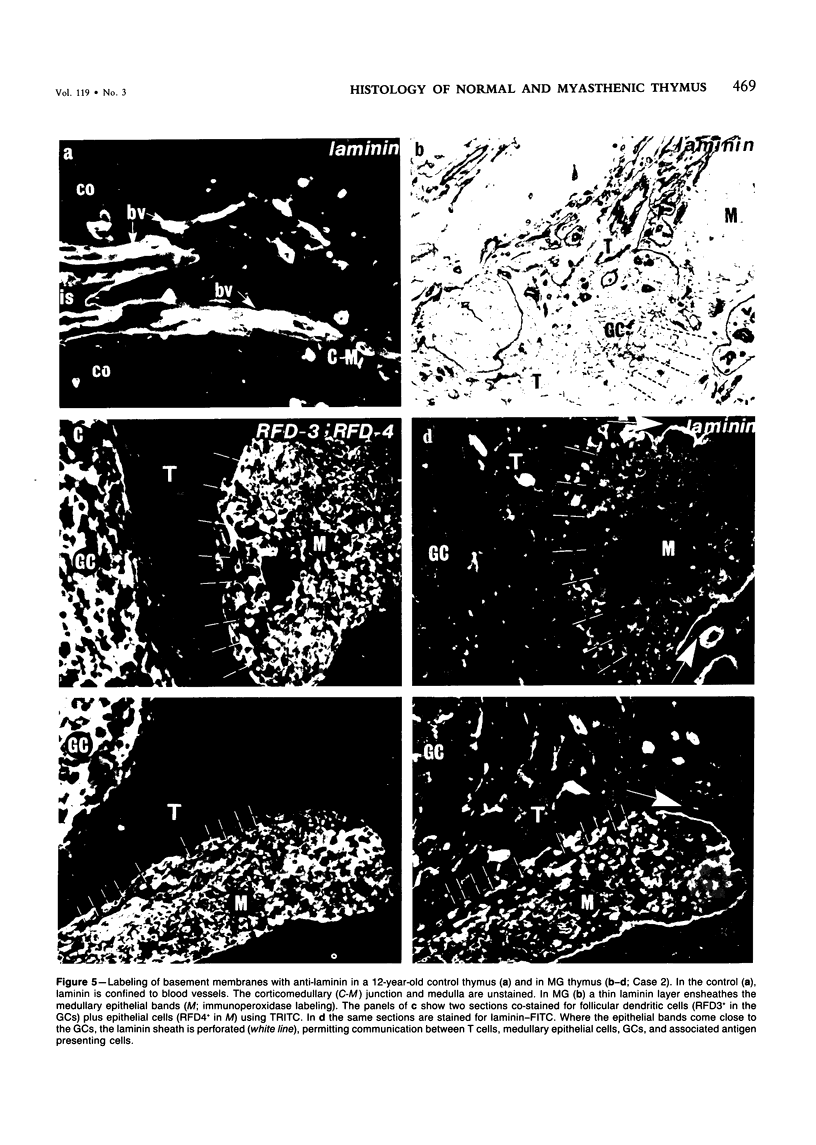
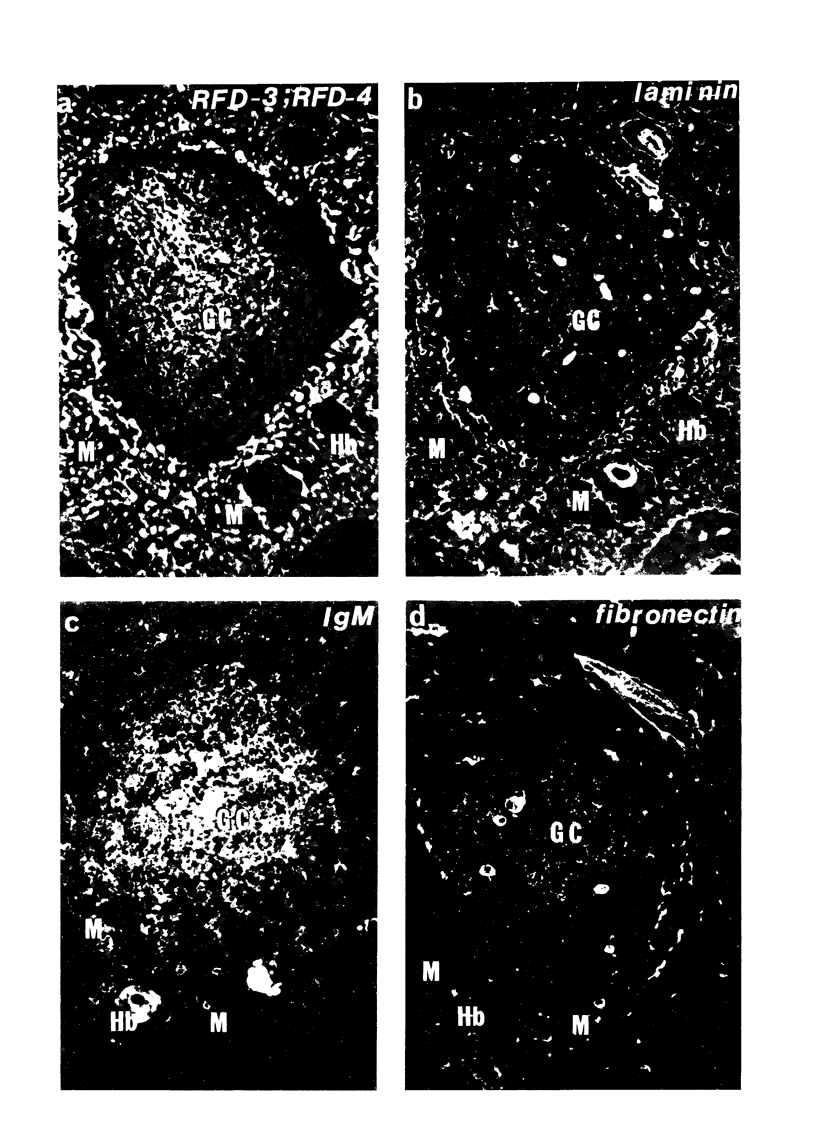
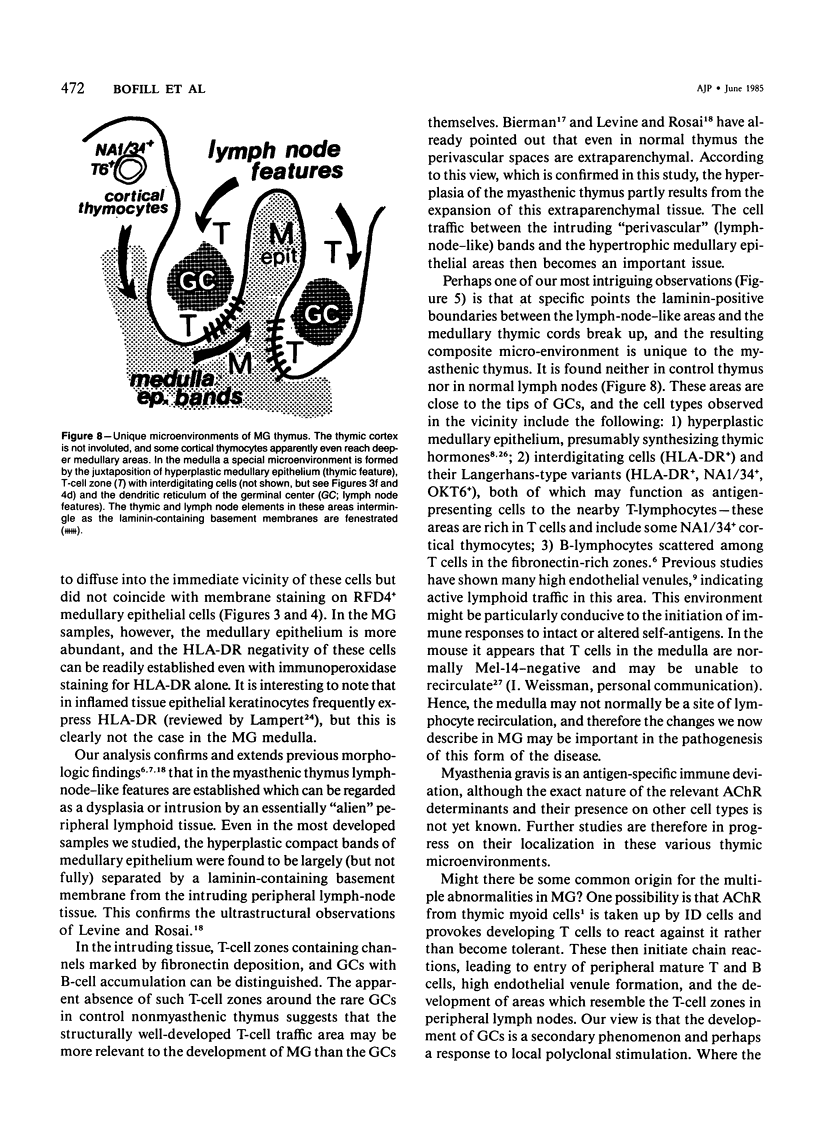
The disposition of epithelial cells and extracellular matrix, in the thymus of 8 cases of myasthenia gravis (MG) and in controls (over a wide age range) was studied. In the controls, the subcapsular epithelium was strongly Leu-7-positive in the fetus, negative in childhood, and positive again in adults. Another antibody, RFD4, also labeled the subcapsular epithelium in childhood and adults, but not fetal samples. The samples from MG cases showed the same staining pattern as adult control samples. The medullary epithelium was also RFD4+, and at all ages. The most striking changes in the advanced cases of MG were the unusual arrangement and hypertrophic appearance of medullary epithelial cell areas, separated by laminin-positive basement membranes from the alternating multiple bands of peripheral lymph-node-like areas. The latter had regions resembling the paracortex of lymph nodes as well as germinal centers (GCs). The T-cell zones contained heavy deposits of fibronectin. These T-cell zones were unique to the thymus in MG and were absent in the two normal thymic samples with isolated GCs. In MG the laminin-containing basement membrane, which separated the medullary epithelial and peripheral lymph-node-like areas, was fenestrated at circumscribed points closest to the GCs, thus apparently permitting communication among the medullary epithelium, the T-cell zones, the GCs and the associated antigen-presenting cells. Large numbers of interdigitating cells and some lymphocytes of cortical thymocyte phenotype were also found at these special sites, where opportunities for autosensitization may persist in MG.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Bearman R. M., Bensch K. G., Levine G. D. The normal human thymic vasculature: an ultrastructural study. Anat Rec. 1975 Dec;183(4):485–497. doi: 10.1002/ar.1091830402. [DOI] [PubMed] [Google Scholar]
- Bofill M., Janossy G., Janossa M., Burford G. D., Seymour G. J., Wernet P., Kelemen E. Human B cell development. II. Subpopulations in the human fetus. J Immunol. 1985 Mar;134(3):1531–1538. [PubMed] [Google Scholar]
- Cordier A. C., Heremans J. F. Nude mouse embryo: ectodermal nature of the primordial thymic defect. Scand J Immunol. 1975;4(2):193–196. doi: 10.1111/j.1365-3083.1975.tb02616.x. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Engel W. K., McClure J. E., Goldstein A. L., Askanas V. Immunocytochemical localization of thymosin-alpha 1 in thymic epithelial cells of normal and myasthenia gravis patients and in thymic cultures. J Neurol Sci. 1981 May;50(2):239–247. doi: 10.1016/0022-510x(81)90170-2. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Riley L. K., Coleman M. S., Cibull M. L., Fuller S. A., Todd E. Expression of terminal deoxynucleotidyl transferase in human thymus during ontogeny and development. J Immunol. 1983 Jul;131(1):195–200. [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Schlesinger D. H. Thymopoietin and myasthenia gravis: neostigmine-responsive neuromuscular block produced in mice by a synthetic peptide fragment of thymopoietin. Lancet. 1975 Aug 9;2(7928):256–259. doi: 10.1016/s0140-6736(75)90966-6. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Shimizu K., Eisenbarth G. S. Identification of human and rodent thymic epithelium using tetanus toxin and monoclonal antibody A2B5. J Clin Invest. 1983 Jan;71(1):9–14. doi: 10.1172/JCI110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Bollum F. J., Granger S., Pizzolo G., Bradstock K. F., Wong L., McMichael A., Ganeshaguru K., Hoffbrand A. V. The human thymic microenvironment: an immunohistologic study. J Immunol. 1980 Jul;125(1):202–212. [PubMed] [Google Scholar]
- Jenkinson E. J., Van Ewijk W., Owen J. J. Major histocompatibility complex antigen expression on the epithelium of the developing thymus in normal and nude mice. J Exp Med. 1981 Feb 1;153(2):280–292. doi: 10.1084/jem.153.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Thymic muscle cells bear acetylcholine receptors: possible relation to myasthenia gravis. Science. 1977 Jan 7;195(4273):74–75. doi: 10.1126/science.831257. [DOI] [PubMed] [Google Scholar]
- Lampert I. A. Expression of HLA-DR (Ia like) antigen on epidermal keratinocytes in human dermatoses. Clin Exp Immunol. 1984 Jul;57(1):93–100. [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis J., Willcox N., Calder L. Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. N Engl J Med. 1981 Nov 26;305(22):1313–1318. doi: 10.1056/NEJM198111263052203. [DOI] [PubMed] [Google Scholar]
- Osserman K. E., Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med. 1971 Nov-Dec;38(6):497–537. [PubMed] [Google Scholar]
- Scadding G. K., Vincent A., Newsom-Davis J., Henry K. Acetylcholine receptor antibody synthesis by thymic lymphocytes: correlation with thymic histology. Neurology. 1981 Aug;31(8):935–943. doi: 10.1212/wnl.31.8.935. [DOI] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Söderström N., Axelsson J. A., Hagelqvist E. Postcapillary venules of the lymph node type in the thymus in myasthenia. Lab Invest. 1970 Nov;23(5):451–458. [PubMed] [Google Scholar]
- Thomas J. A., Willcox H. N., Newsom-Davis J. Immunohistological studies of the thymus in myasthenia gravis. Correlation with clinical state and thymocyte culture responses. J Neuroimmunol. 1982 Dec;3(4):319–335. doi: 10.1016/0165-5728(82)90035-2. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Smolira M. A., Hodges G. M., Goodman S. L., Livingston D. C. Cell surface distribution of fibronectin in cultures of fibroblasts and bladder derived epithelium: SEM-immunogold localization compared to immunoperoxidase and immunofluorescence.. J Microsc. 1981 Aug;123(Pt 2):227–236. doi: 10.1111/j.1365-2818.1981.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Vezzoni P., Fiacchino F., Clerici L., Sghirlanzoni A., Cerrato D., Peluchetti D., Lucchini R., Raineri M., Cornelio F. Studies on terminal deoxynucleotidyl transferase and adenosine deaminase in myasthenic thymus. J Neuroimmunol. 1984 Sep-Oct;6(6):427–433. doi: 10.1016/0165-5728(84)90067-5. [DOI] [PubMed] [Google Scholar]
- Willcox H. N., Newsom-Davis J., Calder L. R. Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol. 1983 Nov;54(2):378–386. [PMC free article] [PubMed] [Google Scholar]