Abstract
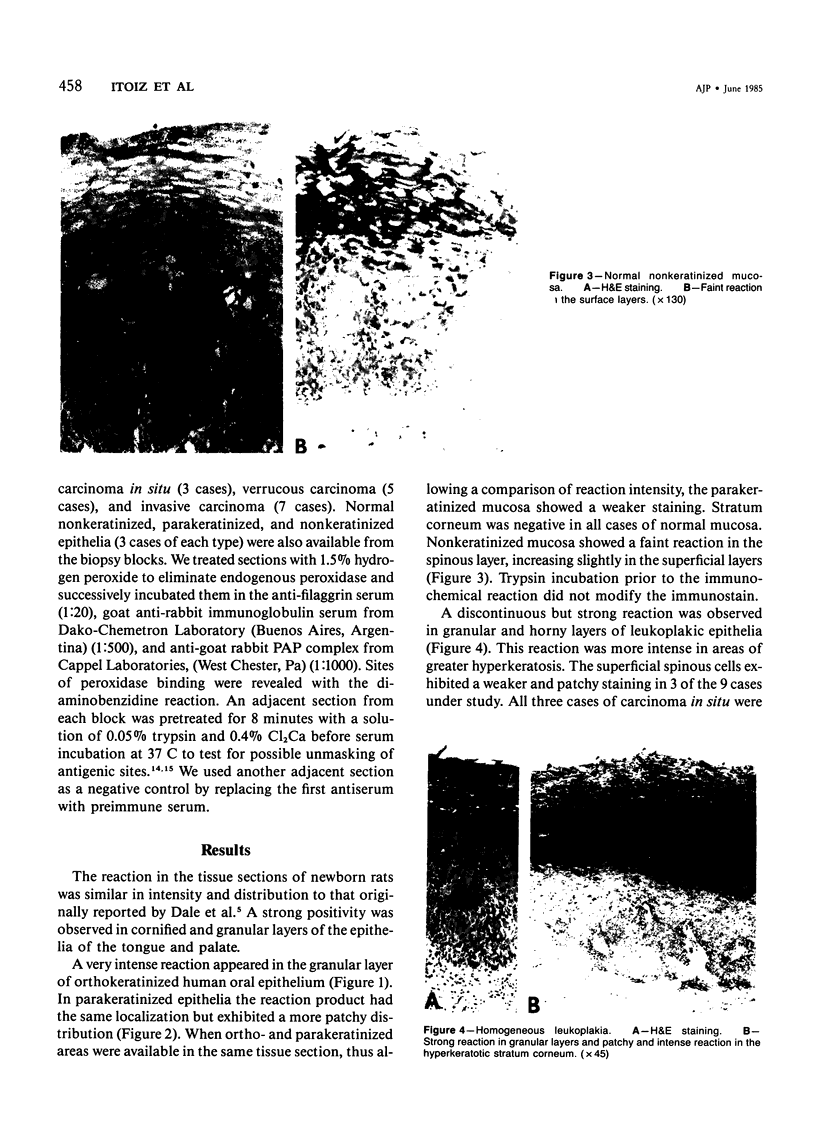
The distribution pattern of filaggrin in lesions of human oral mucosa was studied with the use of an anti-filaggrin serum raised in rabbits. A peroxidase-antiperoxidase method for the detection of filaggrin was applied to specimens from 9 cases of leukoplakia, 5 cases of verrucous carcinomas, 2 cases of carcinoma in situ, and 5 cases of invasive carcinoma. Areas of normal mucosa with different stages of keratinization were available in the same biopsy specimens. The granular layer of normal orthokeratinized epithelium was positive, whereas the horny layer was negative. Parakeratinized and nonkeratinized epithelia stained less than orthokeratinized epithelium. In leukoplakia and verrucous carcinoma, the reaction was irregular both in the granular and the cornified layers. Carcinoma in situ had a virtually negative reaction, and invasive carcinoma exhibited a slight positive reaction in the more differentiated areas. The immunohistochemical demonstration of altered filaggrin patterns in oral lesions correlates well with the degree of epithelial dysplasia and could be a helpful tool in grading white lesions and neoplasms of the oral mucosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. D., Walker G. K., Bernstein I. A. Histidine-rich proteins as molecular markers of epidermal differentiation. J Biol Chem. 1978 Aug 25;253(16):5861–5868. [PubMed] [Google Scholar]
- Bánóczy J., Juhász J., Albrecht M. Ultrastructure of different clinical forms of oral leukoplakia. J Oral Pathol. 1980 Jan;9(1):41–53. doi: 10.1111/j.1600-0714.1980.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Ling S. Y. Evidence of a precursor form of stratum corneum basic protein in rat epidermis. Biochemistry. 1979 Aug 7;18(16):3539–3546. doi: 10.1021/bi00583a016. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Ling S. Y. Immunologic cross-reaction of stratum corneum basic protein and a keratohyalin granule protein. J Invest Dermatol. 1979 May;72(5):257–261. doi: 10.1111/1523-1747.ep12531715. [DOI] [PubMed] [Google Scholar]
- Dale B. A. Purification and characterization of a basic protein from the stratum corneum of mammalian epidermis. Biochim Biophys Acta. 1977 Mar 28;491(1):193–204. doi: 10.1016/0005-2795(77)90055-1. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Thompson W. B., Stern I. B. Distribution of histidine-rich basic protein, a possible keratin matrix protein, in rat oral epithelium. Arch Oral Biol. 1982;27(7):535–545. doi: 10.1016/0003-9969(82)90067-x. [DOI] [PubMed] [Google Scholar]
- Itoiz M. E., Lanfranchi H. E., Gimenez-Conti I. B., Conti C. J. Immunohistochemical demonstration of keratins in oral mucosa lesions. Acta Odontol Latinoam. 1984;1(1):47–51. [PubMed] [Google Scholar]
- Klein-Szanto A. J., Barr R. J., Reiners J. J., Jr, Mamrack M. D. Filaggrin distribution in keratoacanthomas and squamous cell carcinoma. Arch Pathol Lab Med. 1984 Nov;108(11):888–890. [PubMed] [Google Scholar]
- Klein-Szanto A. J., Bánóczy J., Schroeder H. E. Metaplastic conversion of the differentiation pattern in oral epithelia affected by leukoplakia simplex. A stereologic study. Pathol Eur. 1976;11(3):189–210. [PubMed] [Google Scholar]
- Lonsdale-Eccles J. D., Haugen J. A., Dale B. A. A phosphorylated keratohyalin-derived precursor of epidermal stratum corneum basic protein. J Biol Chem. 1980 Mar 25;255(6):2235–2238. [PubMed] [Google Scholar]
- Löning T., Burkhardt A. Dyskeratosis in human and experimental oral precancer and cancer. An immunohistochemical and ultrastructural study in men, mice and rats. Arch Oral Biol. 1982;27(5):361–366. doi: 10.1016/0003-9969(82)90144-3. [DOI] [PubMed] [Google Scholar]
- Mamrack M. D., Klein-Szanto A. J., Reiners J. J., Jr, Slaga T. J. Alteration in the distribution of the epidermal protein filaggrin during two-stage chemical carcinogenesis in the SENCAR mouse skin. Cancer Res. 1984 Jun;44(6):2634–2641. [PubMed] [Google Scholar]
- Mepham B. L., Frater W., Mitchell B. S. The use of proteolytic enzymes to improve immunoglobulin staining by the PAP technique. Histochem J. 1979 May;11(3):345–357. doi: 10.1007/BF01005033. [DOI] [PubMed] [Google Scholar]
- Scott I. R., Harding C. R. Studies on the synthesis and degradation of a high molecular weight, histidine-rich phosphoprotein from mammalian epidermis. Biochim Biophys Acta. 1981 Jun 29;669(1):65–78. doi: 10.1016/0005-2795(81)90224-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Cantieri J. S., Teller D. C., Lonsdale-Eccles J. D., Dale B. A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]