Abstract
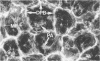
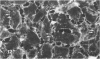
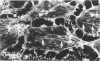
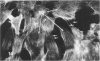
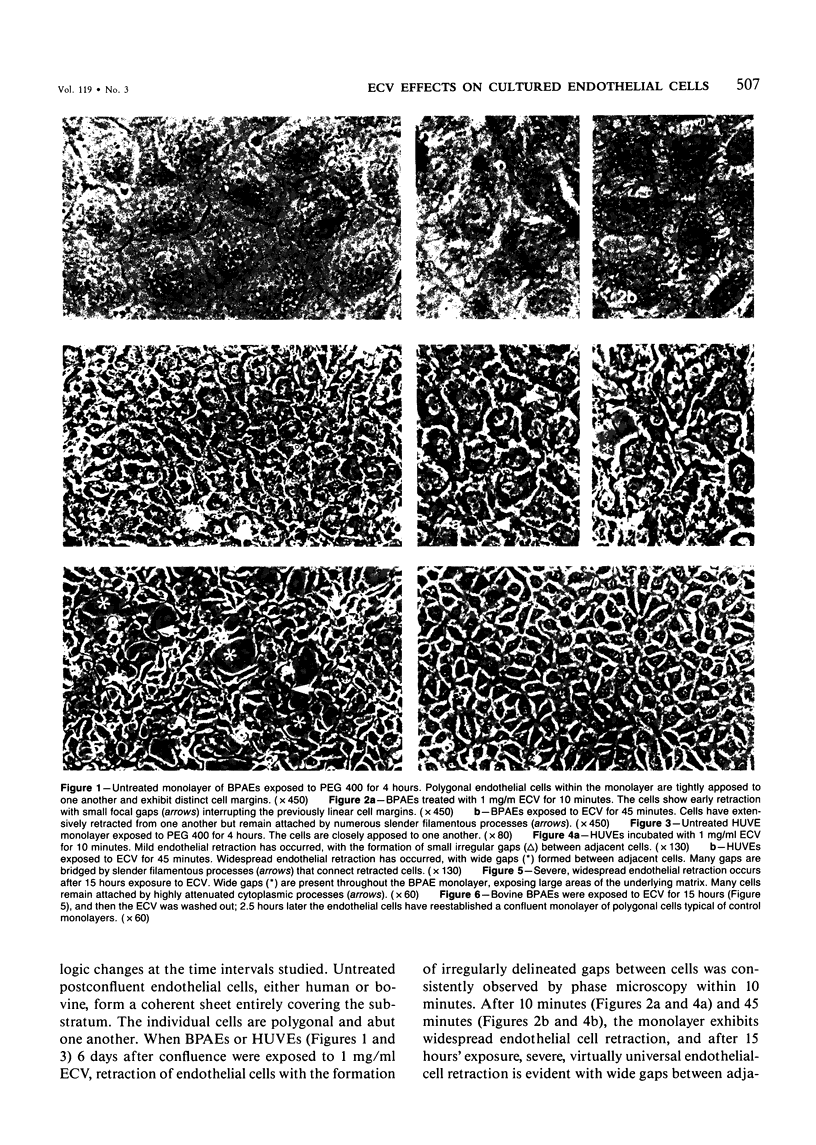
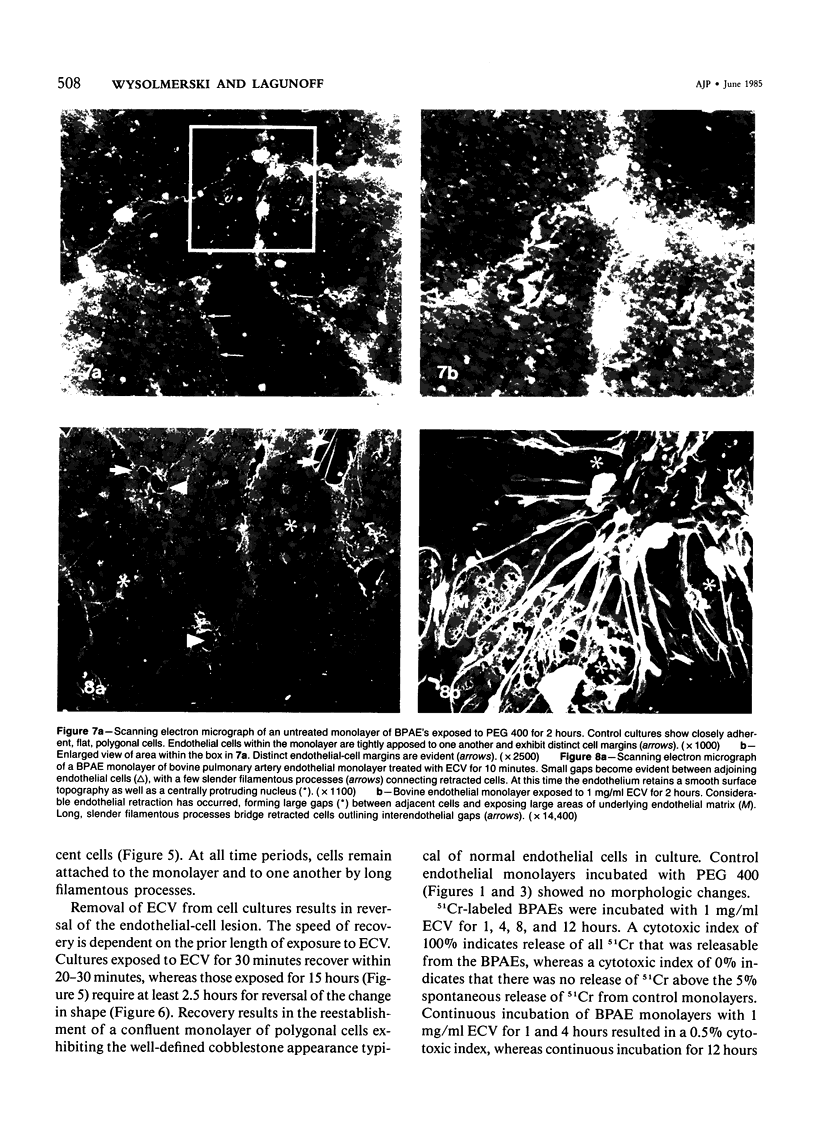
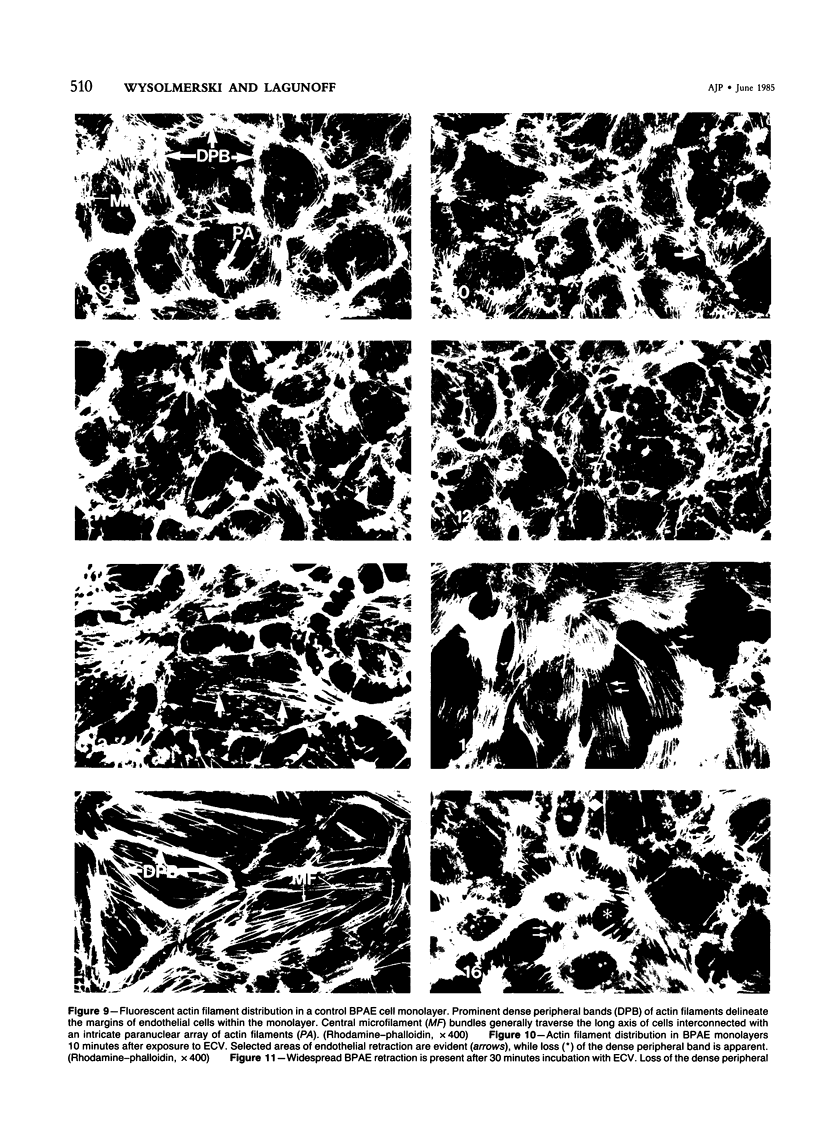
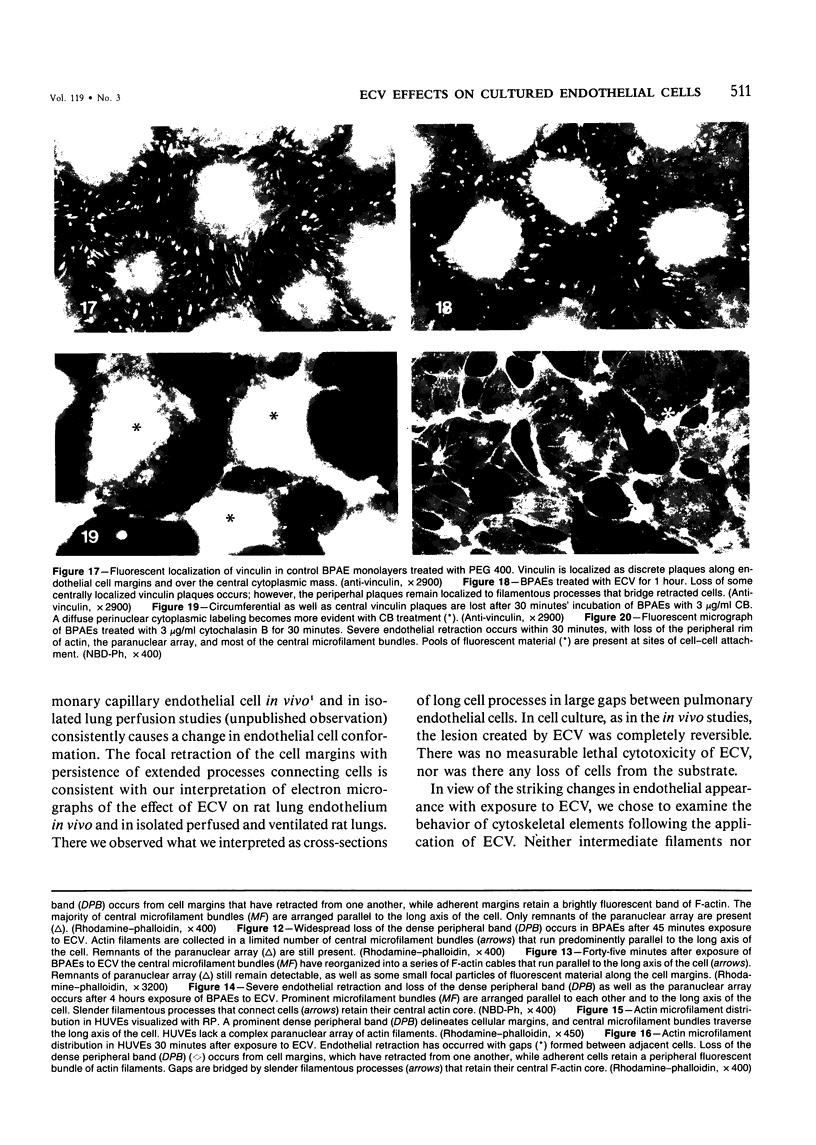
Ethchlorvynol (ECV), an agent which produces reversible pulmonary edema, was studied for its effects on cultured bovine pulmonary artery endothelial cell (BPAE) and human umbilical vein endothelial cell (HUVE) monolayers. Endothelial cell monolayers 6 days post-confluent were treated with 1 mg/ml ECV for time intervals of from 5 minutes to 15 hours. ECV treatment caused a mild endothelial cell retraction evident at 10 minutes which increased in severity with increasing duration of exposure to ECV. Retraction of endothelial cells resulted in the formation of irregularly delineated gaps between cells, which remained attached to one another by slender filamentous processes. Despite the severity of the endothelial cell lesion, no cell lysis or cell detachment from the substratum occurred. Furthermore, removal of ECV from cell cultures resulted in the reversal of the endothelial cell lesion. Cytochemical distribution of actin microfilaments in control monolayers localized to a dense peripheral band of actin filaments and to a set of interconnected central microfilaments oriented in general parallel to the long axis of the cell. Endothelial cells treated with ECV for as little as 10 minutes showed a loss of F-actin from the dense peripheral band of microfilaments progressing until the dense peripheral band was entirely lost after 4 hours' exposure to ECV. By 4 hours central microfilaments had reorganized into a prominent series of microfilament bundles aligned parallel to each other and to the long axis of the cell. For investigation of a possible loss of attachment sites of actin filaments as the basis for the lesion, the localization of vinculin was examined in control and ECV-treated BPAE monolayers. After 2 hours' exposure to ECV, vinculin localization within monolayers was affected little, if at all. No effects of ECV on intermediate filaments were observed either. It is proposed that the dense peripheral band of actin bundles is important in maintaining well-spread endothelial cells in monolayers and that ECV acts to destroy the integrity of this structure. It is further proposed that a reaction of endothelial cells to ECV in vivo analogous to that seen in tissue culture accounts for the production of pulmonary edema by creating gaps between cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio P. J., Smith J. R. Expression of angiotensin-converting enzyme activity in cultured pulmonary artery endothelial cells. J Cell Physiol. 1981 Sep;108(3):337–345. doi: 10.1002/jcp.1041080307. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Greenburg G., Gospodarowicz D. Synthesis and distribution of cytoskeletal elements in endothelial cells as a function of cell growth and organization. J Cell Physiol. 1982 Feb;110(2):129–141. doi: 10.1002/jcp.1041100205. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R. T., Harlan J. M., Harker L. A., Striker G. E. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980 Apr 1;18(1-2):113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. In vitro reendothelialization of a single-cell wound. Role of microfilament bundles in rapid lamellipodia-mediated wound closure. Lab Invest. 1984 Jul;51(1):75–81. [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D., Dahms T. Ethchlorvynol-induced pulmonary edema in rats. An ultrastructural study. Am J Pathol. 1984 Jun;115(3):447–457. [PMC free article] [PubMed] [Google Scholar]