Abstract
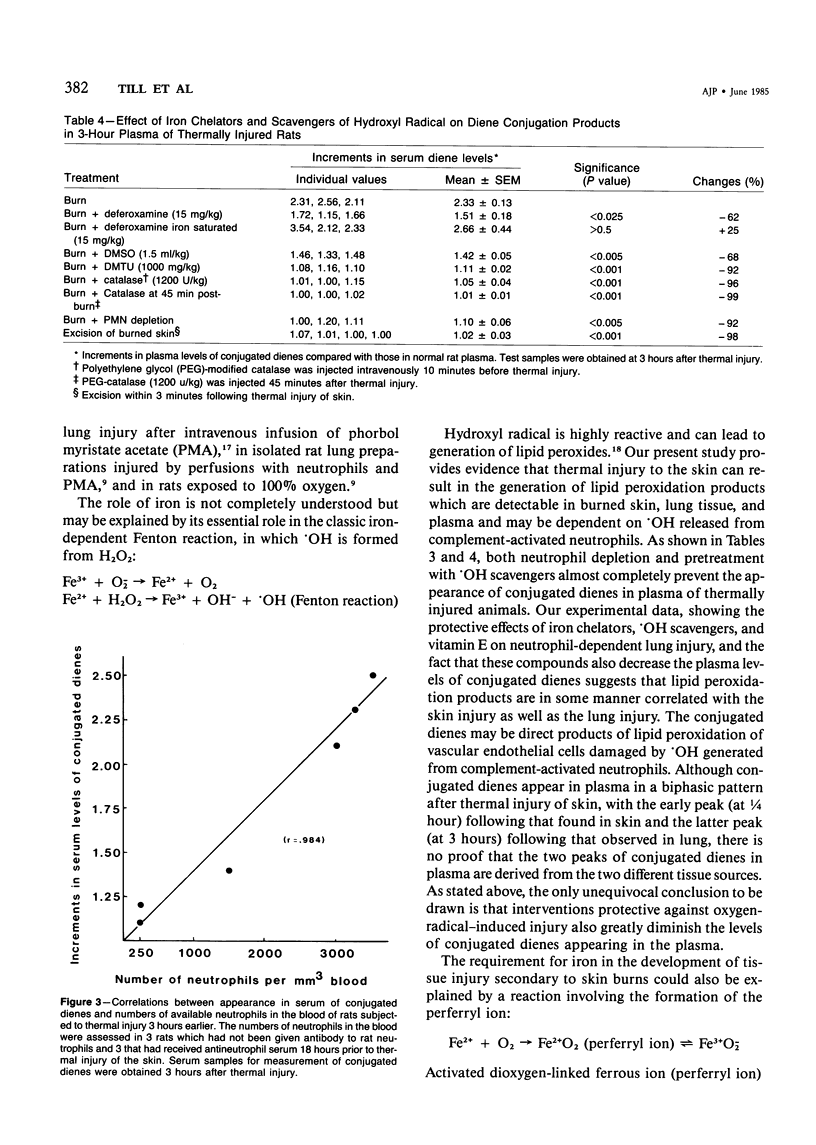
The authors have previously shown that thermal injury to the skin of rats results in the development of acute lung injury that is susceptible to systemic treatment of animals with catalase and dependent on the presence of neutrophils. The current studies have been expanded for exploration of the nature of the neutrophil-derived oxygen products responsible for the lung injury and have also focused on evidence of the appearance of products of lipid peroxidation (conjugated dienes). With respect to the former, treatment of rats with iron chelators (deferoxamine mesylate, 2,3-dihydroxybenzoic acid), with scavengers of hydroxyl radical (dimethyl sulfoxide, dimethyl thiourea, sodium benzoate), or with vitamin E affords a significant degree of protection from acute lung injury as assessed by changes in lung vascular permeability and by morphologic parameters. These data suggest that lung vascular injury after thermal trauma of the skin is related to the generation by neutrophils of the hydroxyl radical. Conjugated dienes have been demonstrated to appear sequentially both in the burned skin (at 1/4 hour) and in the lungs (at 2 hours), as well as in the plasma (with peaks at 1/2 and at 3 hours) after thermal injury. The appearance of the conjugated dienes in plasma at the two intervals of time is greatly diminished if animals are pretreated with the iron chelator deferoxamine, with catalase, or with scavengers of hydroxyl radical. Furthermore, the appearance of conjugated dienes in plasma at 30 minutes and 3 hours is significantly diminished if animals are depleted of neutrophils, complement-depleted, or the burned skin is excised immediately after thermal injury. These data indicate a linkage between thermal trauma of skin, secondary injury of lung, and appearance in plasma and tissues of products of lipid peroxidation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano J. H., Grady R. W., Cerami A. The identification of 2, 3-dihydroxybenzoic acid as a potentially useful iron-chelating drug. J Pharmacol Exp Ther. 1974 Sep;190(3):570–575. [PubMed] [Google Scholar]
- Green J. Vitamin E and the biological antioxidant theory. Ann N Y Acad Sci. 1972 Dec 18;203:29–44. doi: 10.1111/j.1749-6632.1972.tb27853.x. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- Nishigaki I., Hagihara M., Hiramatsu M., Izawa Y., Yagi K. Effect of thermal injury on lipid peroxide levels of rat. Biochem Med. 1980 Oct;24(2):185–189. doi: 10.1016/0006-2944(80)90010-1. [DOI] [PubMed] [Google Scholar]
- Noronha-Dutra A. A., Steen E. M. Lipid peroxidation as a mechanism of injury in cardiac myocytes. Lab Invest. 1982 Oct;47(4):346–353. [PubMed] [Google Scholar]
- PLACER Z., VESELKOVA A., RATH R. KINETIK DES MALONDIALDEHYDES IM ORGANISMUS. Experientia. 1965 Jan 15;21:19–20. doi: 10.1007/BF02136359. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966 Jan;15(1 Pt 1):132–148. [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., BRAND F. C. The determination of total lipides in blood serum. J Biol Chem. 1955 Mar;213(1):69–76. [PubMed] [Google Scholar]
- Till G. O., Beauchamp C., Menapace D., Tourtellotte W., Jr, Kunkel R., Johnson K. J., Ward P. A. Oxygen radical dependent lung damage following thermal injury of rat skin. J Trauma. 1983 Apr;23(4):269–277. doi: 10.1097/00005373-198304000-00001. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Bodaness R. S., Gery I., Kinoshita J. H. Effects of lipid peroxidation products on the rat lens in organ culture: a possible mechanism of cataract initiation in retinal degenerative disease. Arch Biochem Biophys. 1983 Aug;225(1):149–156. doi: 10.1016/0003-9861(83)90018-8. [DOI] [PubMed] [Google Scholar]