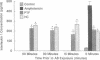Abstract
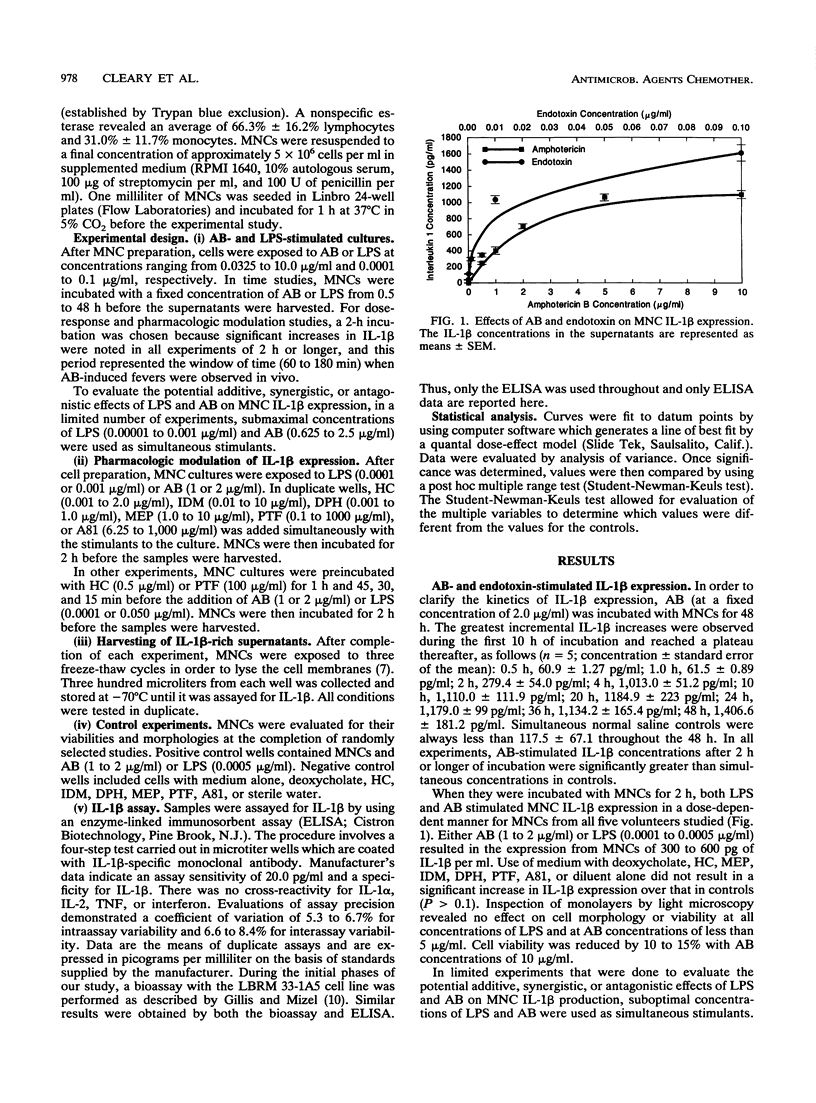
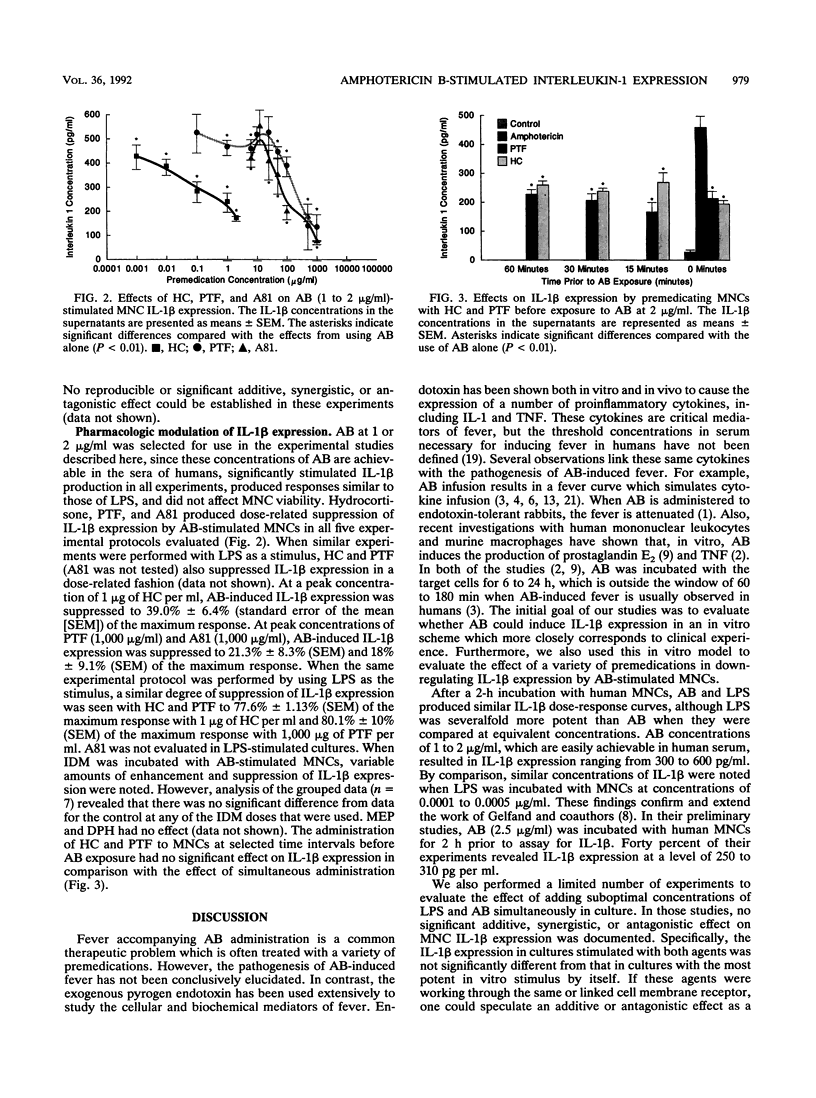
Fever and chills occur frequently with amphotericin B (AB) administration, but the mechanism that causes these reactions has not been definitively established. A variety of proinflammatory cytokines, including interleukin-1 (IL-1) and tumor necrosis factor, have been shown to be important mediators of fever. In order to clarify the cellular and biochemical responses associated with AB-induced fever, the experiments described sought to (i) establish whether human mononuclear cells exposed to AB in vitro expressed IL-1 beta, (ii) evaluate whether clinically used premedications for fever prophylaxis in AB-treated patients were effective in down-regulating IL-1 beta expression in vitro, (iii) evaluate whether methylxanthine agents with immunomodulatory actions effected in vitro IL-1 beta expression, and (iv) define the dose and time dependency of the modulating effects. Peripheral blood mononuclear cells were isolated by density centrifugation and resuspended to 10(6) cells per ml in culture wells of Linbro plates. When cocultured for 2 h with human mononuclear cells, both Escherichia coli lipopolysaccharide and AB stimulated IL-1 beta expression in a dose-related fashion. AB-induced IL-1 beta expression was suppressed by hydrocortisone (HC), pentoxifylline, and an investigational theobromine, A81-3138, in a linear, dose-related manner. In contrast, indomethacin, meperidine, and diphenhydramine had no effect on IL-1 beta expression. Our in vitro data indicate that serum HC concentrations of greater than 1 to 2 micrograms/ml may be sufficient to modulate IL-1 beta expression. Pentoxifylline and A81-3138 may also be effective in modulating IL-1 beta expression by mononuclear cells at concentrations achievable in serum. These new agents may prove to be effective alternatives to HC or may be added with HC to suppress febrile reactions secondary to AB administration. Clinical studies with pentoxifylline as a premedication for AB seem warranted.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo J. C. Amphotericin B fever in rabbits. Life Sci. 1984 Mar 26;34(13):1249–1252. doi: 10.1016/0024-3205(84)90547-2. [DOI] [PubMed] [Google Scholar]
- Chia J. K., Pollack M. Amphotericin B induces tumor necrosis factor production by murine macrophages. J Infect Dis. 1989 Jan;159(1):113–116. doi: 10.1093/infdis/159.1.113. [DOI] [PubMed] [Google Scholar]
- Cleary J. D., Weisdorf D., Fletcher C. V. Effect of infusion rate on amphotericin B-associated febrile reactions. Drug Intell Clin Pharm. 1988 Oct;22(10):769–772. doi: 10.1177/106002808802201005. [DOI] [PubMed] [Google Scholar]
- Davatelis G., Wolpe S. D., Sherry B., Dayer J. M., Chicheportiche R., Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science. 1989 Feb 24;243(4894 Pt 1):1066–1068. doi: 10.1126/science.2646711. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M. New concepts on the pathogenesis of fever. Rev Infect Dis. 1988 Jan-Feb;10(1):168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Lonnemann G., van der Meer J. W., Dinarello C. A. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin Immunol Immunopathol. 1988 Dec;49(3):424–438. doi: 10.1016/0090-1229(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Shenep J. L., Lott L., Thornton D. Induction of prostaglandin synthesis as the mechanism responsible for the chills and fever produced by infusing amphotericin B. J Infect Dis. 1987 Nov;156(5):784–789. doi: 10.1093/infdis/156.5.784. [DOI] [PubMed] [Google Scholar]
- Gillis S., Mizel S. B. T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Thompson P., Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990 Jul 1;172(1):391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman T., Lee Y. L., Lizzio E. F., Tripathi A. K., Jessop J. J., Taplits M., Abrahamsen T. G., Carter C. S., Puri J. Absence of modulation of monokine production via endogenous cyclooxygenase or 5-lipoxygenase metabolites: MK-886 (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2- dimethylpropanoic acid), indomethacin, or arachidonate fail to alter immunoreactive interleukin-1 beta, or TNF-alpha production by human monocytes in vitro. Clin Immunol Immunopathol. 1991 Mar;58(3):399–408. doi: 10.1016/0090-1229(91)90130-3. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., Ghezzi P., van der Meer J. W., Dinarello C. A. Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990 Jul;162(1):215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]
- Kassis S., Lee J. C., Hanna N. Effects of prostaglandins and cAMP levels on monocyte IL-1 production. Agents Actions. 1989 Jun;27(3-4):274–276. doi: 10.1007/BF01972795. [DOI] [PubMed] [Google Scholar]
- Kern J. A., Lamb R. J., Reed J. C., Elias J. A., Daniele R. P. Interleukin-1-beta gene expression in human monocytes and alveolar macrophages from normal subjects and patients with sarcoidosis. Am Rev Respir Dis. 1988 May;137(5):1180–1184. doi: 10.1164/ajrccm/137.5.1180. [DOI] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Meyer F. A., Yaron I., Mashiah V., Yaron M. Effect of diclofenac on prostaglandin E and hyaluronic acid production by human synovial fibroblasts stimulated with interleukin-1. Br J Clin Pharmacol. 1989 Aug;28(2):193–196. doi: 10.1111/j.1365-2125.1989.tb05416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- North R. J. The action of cortisone acetate on cell-mediated immunity to infection. Suppression of host cell proliferation and alteration of cellular composition of infective foci. J Exp Med. 1971 Dec 1;134(6):1485–1500. doi: 10.1084/jem.134.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Sagone A. L., Balcerzak S. P., Ackerman G. A., LoBuglio A. F. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975 Jan 30;292(5):236–241. doi: 10.1056/NEJM197501302920504. [DOI] [PubMed] [Google Scholar]
- Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985 Nov;76(5):1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Ward P. A., Spengler R. N., Lynch J. P., 3rd, Larrick J., Kunkel S. L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- TYNES B. S., UTZ J. P., BENNETT J. E., ALLING D. W. Reducing amphotericin B reactions. A double-blind study. Am Rev Respir Dis. 1963 Feb;87:264–268. doi: 10.1164/arrd.1963.87.2.264. [DOI] [PubMed] [Google Scholar]
- Ward A., Clissold S. P. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987 Jul;34(1):50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]