Abstract
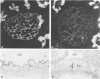
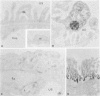
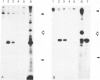
Membranous glomerulonephritis in the mouse can be induced by a single injection of an antiserum against homologous, pronase-digested, renal tubular antigens (TAPron). In indirect immunofluorescence studies on normal mouse and rat kidneys it has now been found that the antiserum reacts strongly with the visceral epithelia of the mouse in a homogeneous pattern, while a faint granular staining is seen in the rat glomerulus against a homogeneous background. After injection in rats, a classic passive Heymann nephritis could be induced. By immunoprecipitation of radiolabeled rat renal brush borders (BB) it could be shown that anti-TAPron antisera contain antibodies to 330-kd and 90-kd BB proteins expressed by rat glomeruli. With the use of two monoclonal antibodies specific for the 330- and 90-kd proteins the homogeneous binding observed in rat and mouse glomeruli could be related to the 90-kd antigen, whereas the coarse irregular staining observed in rat glomeruli was only related to the 330-kd antigen. Immunoglobulins eluted from glomeruli of rats bound to rat glomeruli and reacted only with the 330-kd protein. They did not bind to mouse glomeruli. Discrete localization in coated pits, multivesicular bodies, and endoplasmic reticulum of the visceral epithelia was seen in immunoelectron-microscopy. The results presented thus demonstrate that immune deposits induced in the rat by anti-TAPron antibodies are related to antibodies specific for the 330-kd antigen, ie, the classic Heymann antigen. By contrast, immune deposits observed in the mouse are related to antibodies specific for a 90-kd protein.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann K. J., Tangelder M. M., Lange W. P., Tadema T. M., Koene R. A. Membranous glomerulonephritis in the mouse. Kidney Int. 1983 Sep;24(3):303–312. doi: 10.1038/ki.1983.159. [DOI] [PubMed] [Google Scholar]
- Barabas A. Z., Cornish J., Lannigan R. Passive Heymann nephritis in the rat produced by a heterologous antibody to a heterologous kidney fraction 3 antigen. Br J Exp Pathol. 1982 Dec;63(6):667–670. [PMC free article] [PubMed] [Google Scholar]
- Bertani T., Nolin L., Foidart J., Vandewalle A., Verroust P. The effect of puromycin on subepithelial deposits induced by antibodies directed against tubular antigens: a quantitative study. Eur J Clin Invest. 1979 Dec;9(6):465–472. doi: 10.1111/j.1365-2362.1979.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Chant S., Katz A., Silverman M. Pathogenicity of a highly purified brush border membrane preparation in Heymann nephritis. J Clin Lab Immunol. 1980 Nov;4(3):133–140. [PubMed] [Google Scholar]
- Couser W. G., Salant D. J. In situ immune complex formation and glomerular injury. Kidney Int. 1980 Jan;17(1):1–13. doi: 10.1038/ki.1980.1. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra K., van den Lee R., Greben H. A., Arends A., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. I. The natural history: a histologic and immunohistologic study at the light microscopic and the ultrastructural level. Lab Invest. 1975 Feb;32(2):235–242. [PubMed] [Google Scholar]
- Fleuren G. J., Grond J., Hoedemaeker P. J. The pathogenetic role of free-circulating antibody in autologous immune complex glomerulonephritis. Clin Exp Immunol. 1980 Aug;41(2):205–217. [PMC free article] [PubMed] [Google Scholar]
- Fleuren G. J., vd Lee R., Greben H. A., Van Damme B. J., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. IV. Investigations into the pathogenesis of the model. Lab Invest. 1978 Apr;38(4):496–501. [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Heymann W., Kmetec E. P., Wilson S. G., Hunter J. L., Hackel D. B., Okuda R., Cuppage F. Experimental autoimmune renal disease in rats. Ann N Y Acad Sci. 1965 Jun 30;124(1):310–322. doi: 10.1111/j.1749-6632.1965.tb18966.x. [DOI] [PubMed] [Google Scholar]
- Hoyer J. R., Mauer S. M., Michael A. F. Unilateral renal disease in the rat. I. Clinical, morphologic, and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab Clin Med. 1975 May;85(5):756–768. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malathi P., Preiser H., Fairclough P., Mallett P., Crane R. K. A rapid method for the isolation of kidney brush border membranes. Biochim Biophys Acta. 1979 Jun 13;554(1):259–263. doi: 10.1016/0005-2736(79)90023-3. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nagi A. H., Barabas A. Z., Alexander F., Lannigan R. Production of autologous immune complex glomerulonephritis in rats by injections of heterologous renal tubular antigen. Acta Med Acad Sci Hung. 1971;28(3):249–257. [PubMed] [Google Scholar]
- Neale T. J., Wilson C. B. Glomerular antigens in Heymann's nephritis: reactivity of eluted and circulating antibody. J Immunol. 1982 Jan;128(1):323–330. [PubMed] [Google Scholar]
- Neale T. J., Woodroffe A. J., Wilson C. B. Spontaneous glomerulonephritis in rabbits: role of a glomerular capillary antigen. Kidney Int. 1984 Nov;26(5):701–711. doi: 10.1038/ki.1984.205. [DOI] [PubMed] [Google Scholar]
- Ronco P., Allegri L., Melcion C., Pirotsky E., Appay M. D., Bariety J., Pontillon F., Verroust P. A monoclonal antibody to brush border and passive Heymann nephritis. Clin Exp Immunol. 1984 Feb;55(2):319–332. [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Melcion C., Geniteau M., Ronco E., Reininger L., Galceran M., Verroust P. Production and characterization of monoclonal antibodies against rat brush border antigens of the proximal convoluted tubule. Immunology. 1984 Sep;53(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- The T. H., Feltkamp T. E. Conjugation of fluorescein isothiocyanate to antibodies. II. A reproducible method. Immunology. 1970 Jun;18(6):875–881. [PMC free article] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]