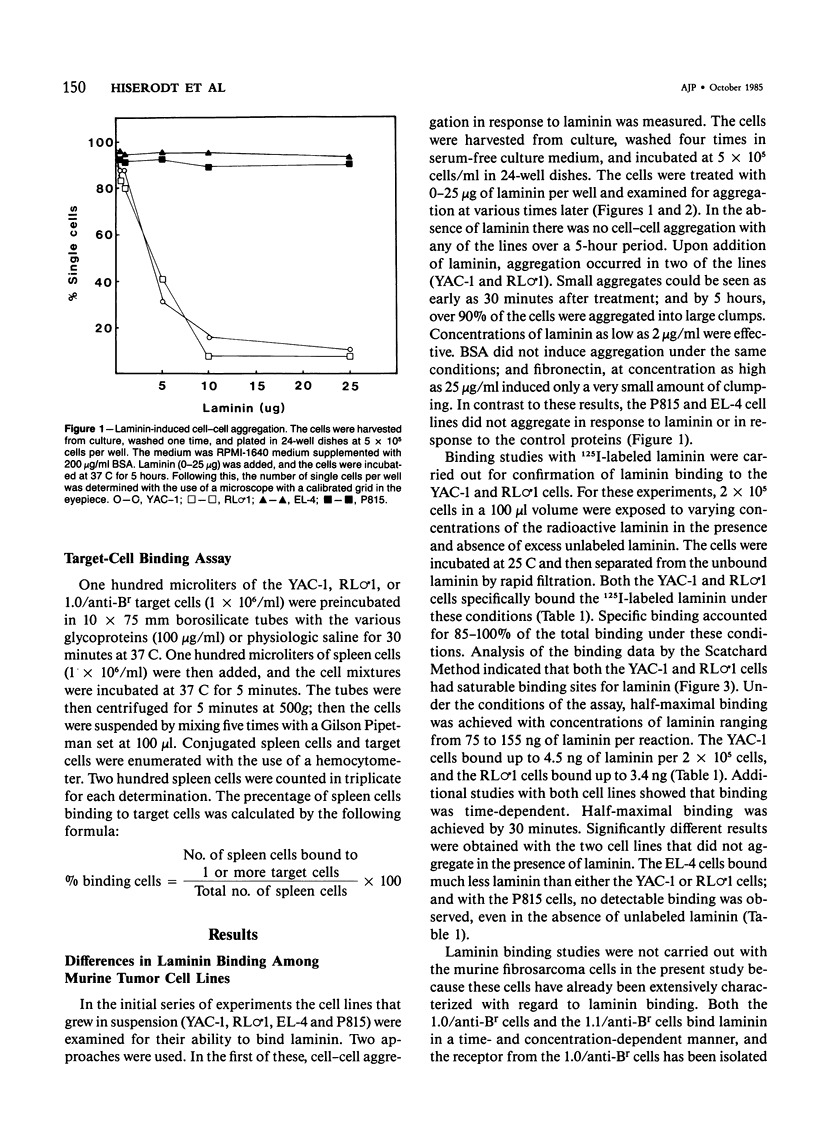
Abstract
Tumor cells sensitive to lysis by murine natural killer (NK) or natural cytotoxic (NC) cells were shown to bind laminin. They bound 125I-labeled laminin in a time- and concentration-dependent manner, and binding of the radioactive laminin was inhibited by excess cold laminin. In the presence of laminin, cell-cell aggregation occurred. Murine tumor cells not sensitive to NK/NC-mediated killing bound much less laminin, and laminin did not induce aggregation of these cells. The addition of exogenous laminin to NK or NC cytotoxicity assays reduced target lysis in a dose-related manner. Reduction of lysis was due to an inability of NK/NC cells to bind to the targets. Target cells pretreated with laminin were reduced in their ability to cold-target compete for NK-mediated lysis of untreated target cells. These effects were unique to laminin. The control proteins (fibronectin and thyroglobulin) had no effect on NK activity. Finally, inhibition of cytolytic activity by laminin appeared to be specific for NK/NC cells. Laminin had no effect on cytolysis mediated by alloimmune cytotoxic T lymphocytes regardless of whether the targets did or did not bind laminin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haller O., Hansson M., Kiessling R., Wigzell H. Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature. 1977 Dec 15;270(5638):609–611. doi: 10.1038/270609a0. [DOI] [PubMed] [Google Scholar]
- Hanna N., Burton R. C. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981 Nov;127(5):1754–1758. [PubMed] [Google Scholar]
- Hanna N., Fidler I. J. Role of natural killer cells in the destruction of circulating tumor emboli. J Natl Cancer Inst. 1980 Oct;65(4):801–809. doi: 10.1093/jnci/65.4.801. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. Surveillance of primitive cells by natural killer cells. Curr Top Microbiol Immunol. 1981;92:107–123. doi: 10.1007/978-3-642-68069-4_7. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Malinoff H. L., McCoy J. P., Jr, Varani J., Wicha M. S. Metastatic potential of murine fibrosarcoma cells is influenced by cell surface laminin. Int J Cancer. 1984 May 15;33(5):651–655. doi: 10.1002/ijc.2910330516. [DOI] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy J. P., Jr, Lloyd R. V., Wicha M. S., Varani J. Identification of a laminin-like substance on the surface of high-malignant murine fibrosarcoma cells. J Cell Sci. 1984 Jan;65:139–151. doi: 10.1242/jcs.65.1.139. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Warner J. F., Dennert G. NK recognition of target structures: is the transferrin receptor the NK target structure? J Immunol. 1984 Oct;133(4):1841–1845. [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Ahrlund-Richter L., Jondal M. Target-effector interaction in the human and murine natural killer system: specificity and xenogeneic reactivity of the solubilized natural killer-target structure complex and its loss in a somatic cell hybrid. J Exp Med. 1979 Sep 19;150(3):471–481. doi: 10.1084/jem.150.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Rosén A., Fenyö E. M., Troy F. A. Target-effector interaction in the natural killer cell system: isolation of target structures. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1405–1409. doi: 10.1073/pnas.76.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J., Bennett M., Mann D., Kumar V. Natural killer cells in mouse lung: surface phenotype, target preference, and response to local influenza virus infection. J Immunol. 1983 Dec;131(6):2699–2704. [PubMed] [Google Scholar]
- Stutman O., Lattime E. C., Figarella E. F. Natural cytotoxic cells against solid tumors in mice: a comparison with natural killer cells. Fed Proc. 1981 Oct;40(12):2699–2704. [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, McCoy J. P., Jr, Shibata S., Maddox D. E., Goldstein I. J., Wicha M. Differential expression of a lamininlike substance by high- and low-metastatic tumor cells. Am J Pathol. 1983 Apr;111(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, Wicha M., Malinoff H., McCoy J. P., Jr Cell surface alpha-D-galactopyranosyl end groups: use as markers in the isolation of murine tumor cell lines with different cancer-causing potentials. J Natl Cancer Inst. 1983 Dec;71(6):1281–1287. [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Hydrolytic enzyme activities, migratory activity, and in vivo growth and metastatic potential of recent tumor isolates. Cancer Res. 1979 Jul;39(7 Pt 1):2376–2380. [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, Durdik J. M., Urdal D., Hakomori S., Henney C. S. Glycolipid expression in lymphoma cell variants: chemical quantity, immunologic reactivity, and correlations with susceptibility to NK cells. J Immunol. 1981 Jan;126(1):1–6. [PubMed] [Google Scholar]



