Abstract
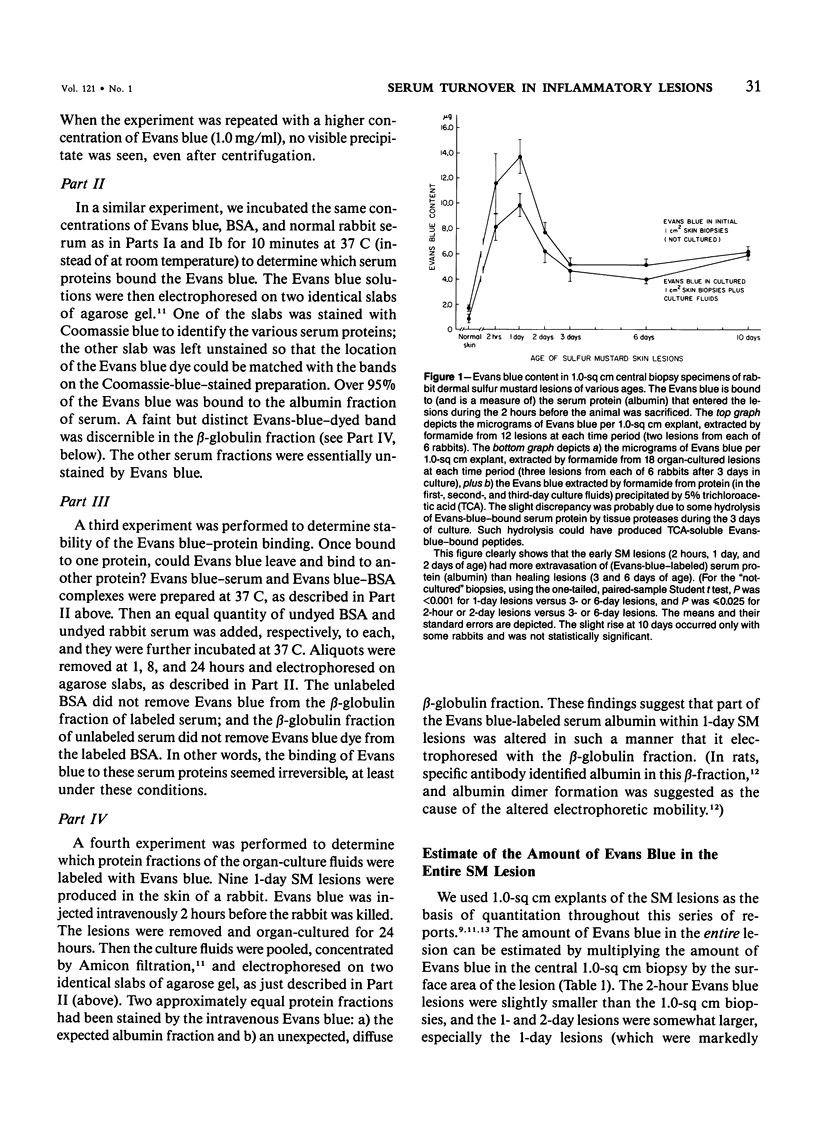
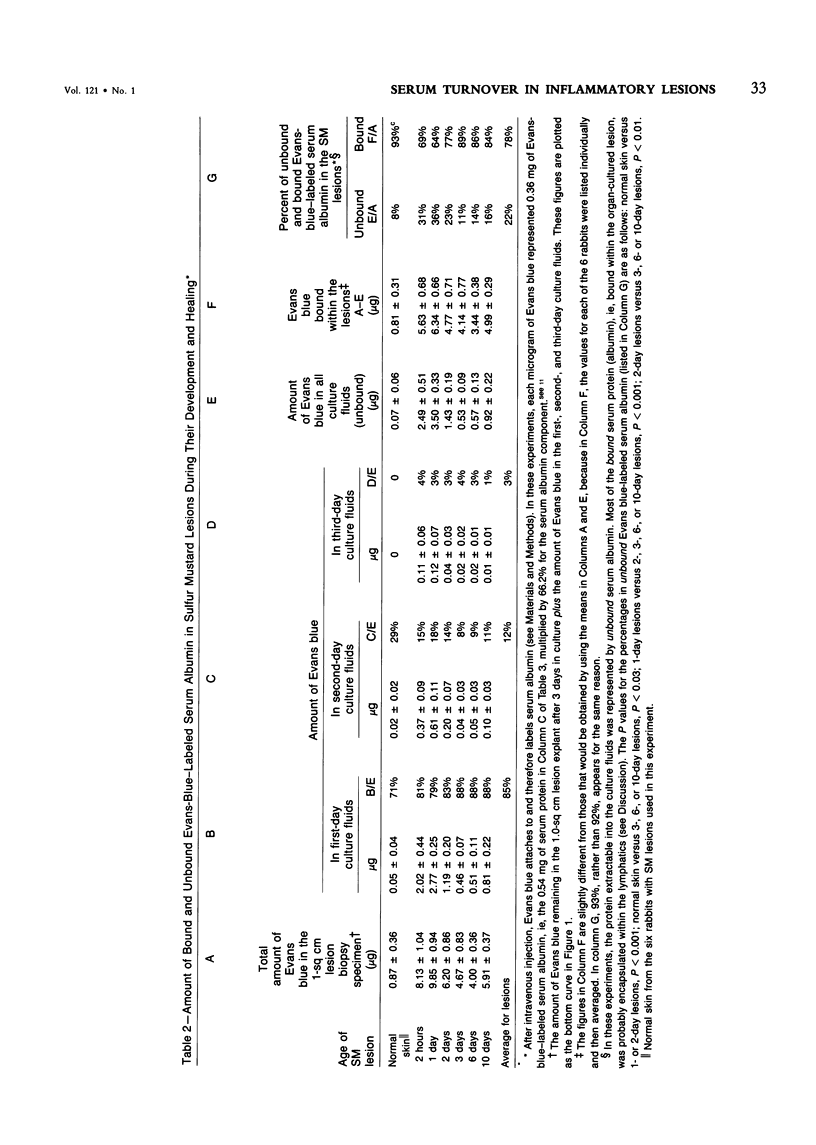
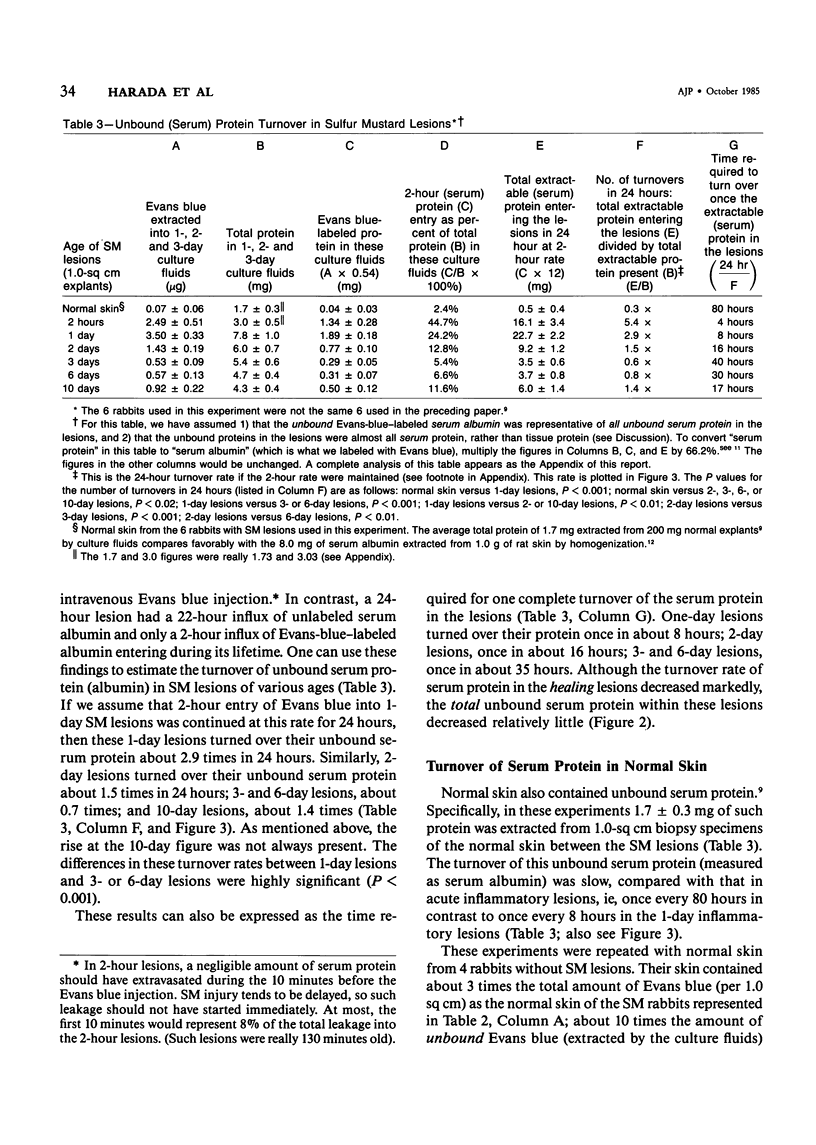
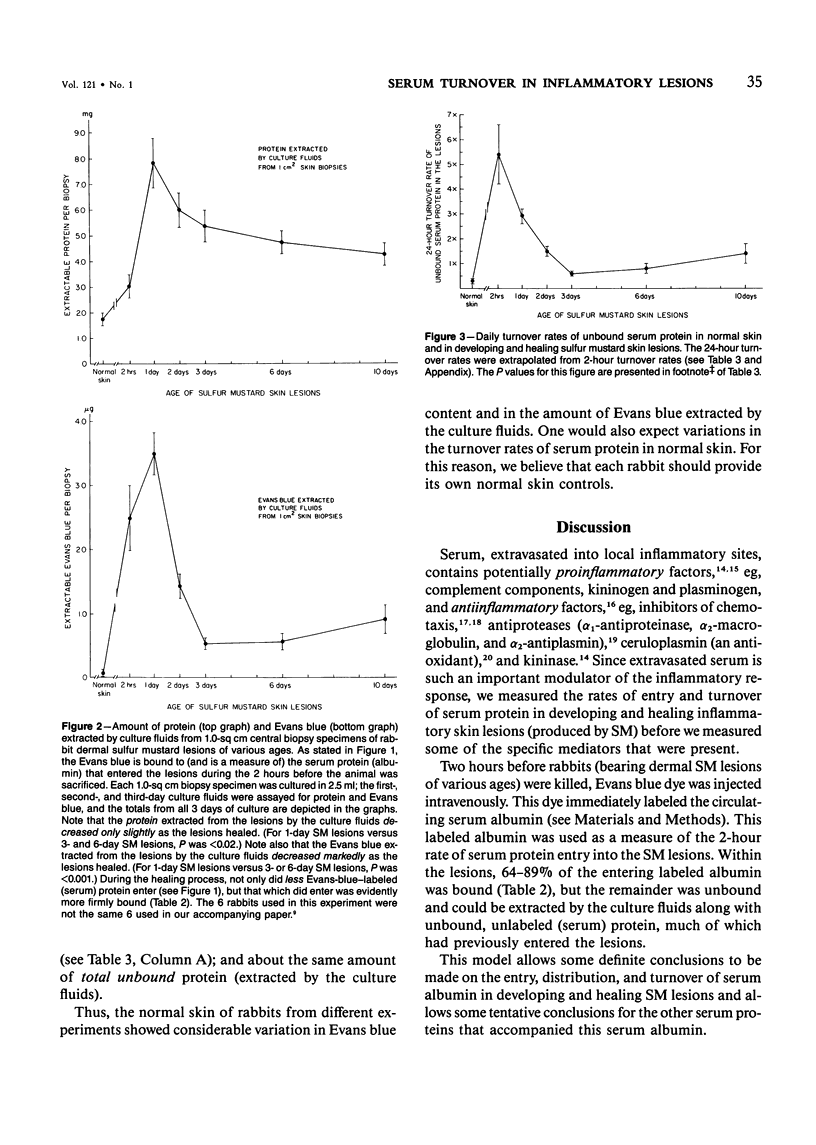
Extravasated serum seems to be the major modulator of the local inflammatory response, because it provides both proinflammatory and antiinflammatory components. This report describes the rates of entry and turnover of extravasated serum protein in dermal inflammatory lesions produced by the military vesicant sulfur mustard (SM). Rabbits, bearing SM skin lesions, were given an intravenous injection of Evans blue dye, so that at the time of sacrifice, 2 hours later, their skin lesions were 2 hours and 1,2,3,6, and 10 days of age. Evans blue labels serum albumin, a representative serum protein. By multiplying the amount of Evans blue contained in the lesions by a factor that converted micrograms of Evans blue into milligrams of serum protein, the authors could estimate the 2-hour rate of entry of serum protein into these lesions. Serum protein in the lesions was both bound and unbound. The unbound protein was extractable from the lesions into the culture fluids, and, electrophoretically, was similar in composition to serum protein. Grossly edematous peak lesions (1 day of age) contained 7.8 mg of unbound serum protein per square centimeter of skin. Healing lesions (6 and 10 days of age) contained about 4.5 mg/sq cm, and normal skin about 1.7 mg/sq cm. Lesions 1 day of age had the highest rate of serum albumin entry, and about 36% of this Evans-blue-labeled protein was unbound, ie, extractable into the culture fluids. Lesions 3 and 6 days of age had a rate of serum albumin entry that was roughly half that of 1-day lesions, and only about 13% of this entering protein was unbound. Normal skin had a very low rate of serum albumin entry, and only 8% of this entering protein was unbound. The turnover rate of the unbound (extractable) serum protein could be estimated from the 2-hour entry rate of the Evans-blue-labeled albumin and the total protein in the culture fluids. In 1-day lesions, about 25% of the serum protein in the culture fluids was protein which had entered during the last 2 hours, so that 100% of this unbound protein should have been replaced once in 8 hours. In contrast, in 3- and 6-day lesions, this unbound serum protein should have been replaced once in about 35 hours, and in normal skin once in 80 hours. Evans-blue-labeled serum albumin continuously entered both the bound and unbound compartments of the SM lesions, even during the healing stages.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colman R. W. Formation of human plasma kinin. N Engl J Med. 1974 Sep 5;291(10):509–515. doi: 10.1056/NEJM197409052911008. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Pula P. J., Liu L. H., Harada S., Tanaka F., Vogt R. F., Jr, Kajiki A., Higuchi K. Inflammatory mediators and modulators released in organ culture from rabbit skin lesions produced in vivo by sulfur mustard. I. Quantitative histopathology; PMN, basophil, and mononuclear cell survival; and unbound (serum) protein content. Am J Pathol. 1985 Oct;121(1):15–27. [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lewis D. A. Endogenous anti-inflammatory factors. Biochem Pharmacol. 1984 Jun 1;33(11):1705–1714. doi: 10.1016/0006-2952(84)90337-x. [DOI] [PubMed] [Google Scholar]
- Maderazo D. G., Ward P. A., Woronick C. L., Quintiliani R. Partial characterization of a cell-directed inhibitor of leukotaxis in human serum. J Lab Clin Med. 1977 Jan;89(1):190–199. [PubMed] [Google Scholar]
- Parving H. H., Hansen J. M., Nielsen S. L., Rossing N., Munck O., Lassen N. A. Mechanisms of edema formation in myxedema--increased protein extravasation and relatively slow lymphatic drainage. N Engl J Med. 1979 Aug 30;301(9):460–465. doi: 10.1056/NEJM197908303010902. [DOI] [PubMed] [Google Scholar]
- Rodbard S., Feldman P. Functional anatomy of the lymphatic fluids and pathways. Lymphology. 1975 Jun;8(2):49–56. [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Schreiber S. S. Extravascular albumin. N Engl J Med. 1979 Aug 30;301(9):497–498. doi: 10.1056/NEJM197908303010909. [DOI] [PubMed] [Google Scholar]
- Vogt R. F., Jr, Dannenberg A. M., Jr, Schofield B. H., Hynes N. A., Papirmeister B. Pathogenesis of skin lesions caused by sulfur mustard. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 2):S71–S83. doi: 10.1016/0272-0590(84)90139-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kambara T. A protease-like permeability factor in the guinea pig skin. 1. Partial purification and characterization. Biochim Biophys Acta. 1978 Apr 19;540(1):55–64. doi: 10.1016/0304-4165(78)90434-8. [DOI] [PubMed] [Google Scholar]
- Zweifach B. W., Silberberg A. The interstitial-lymphatic flow system. Int Rev Physiol. 1979;18:215–260. [PubMed] [Google Scholar]


