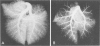
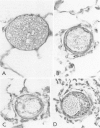
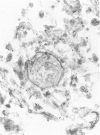
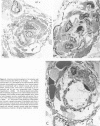
Abstract
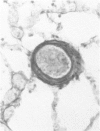
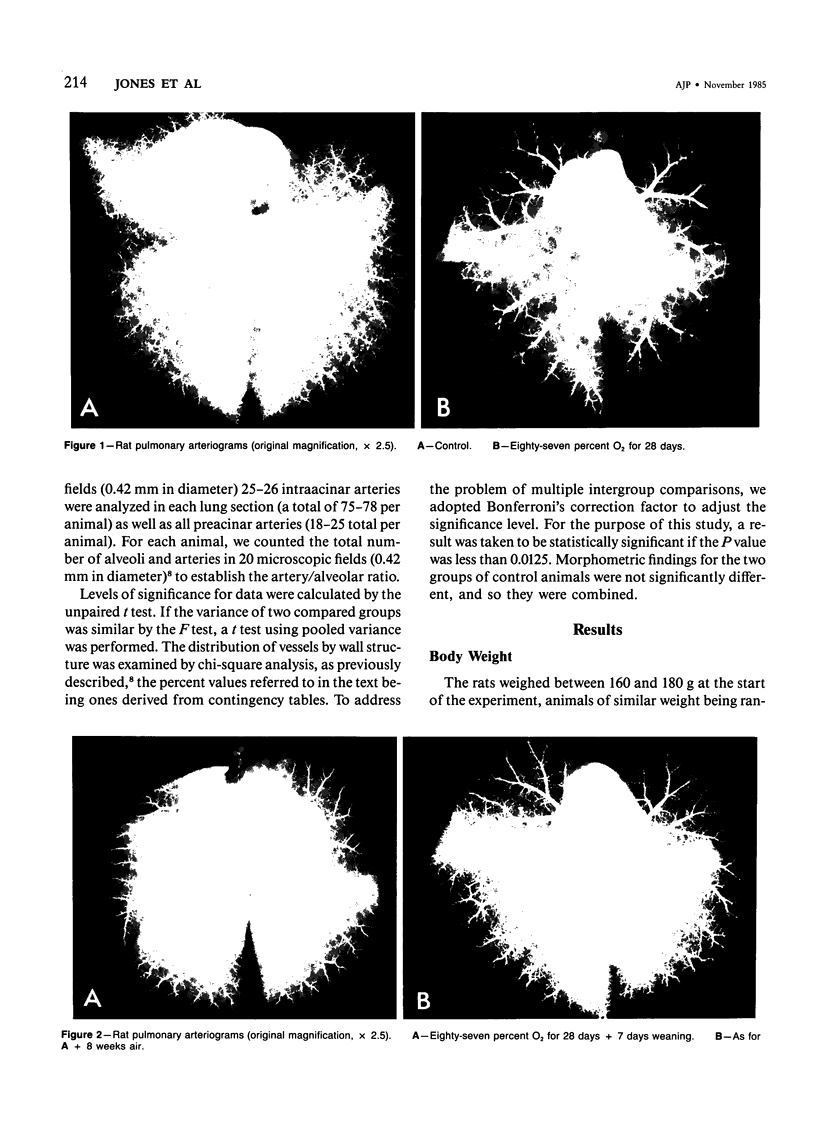
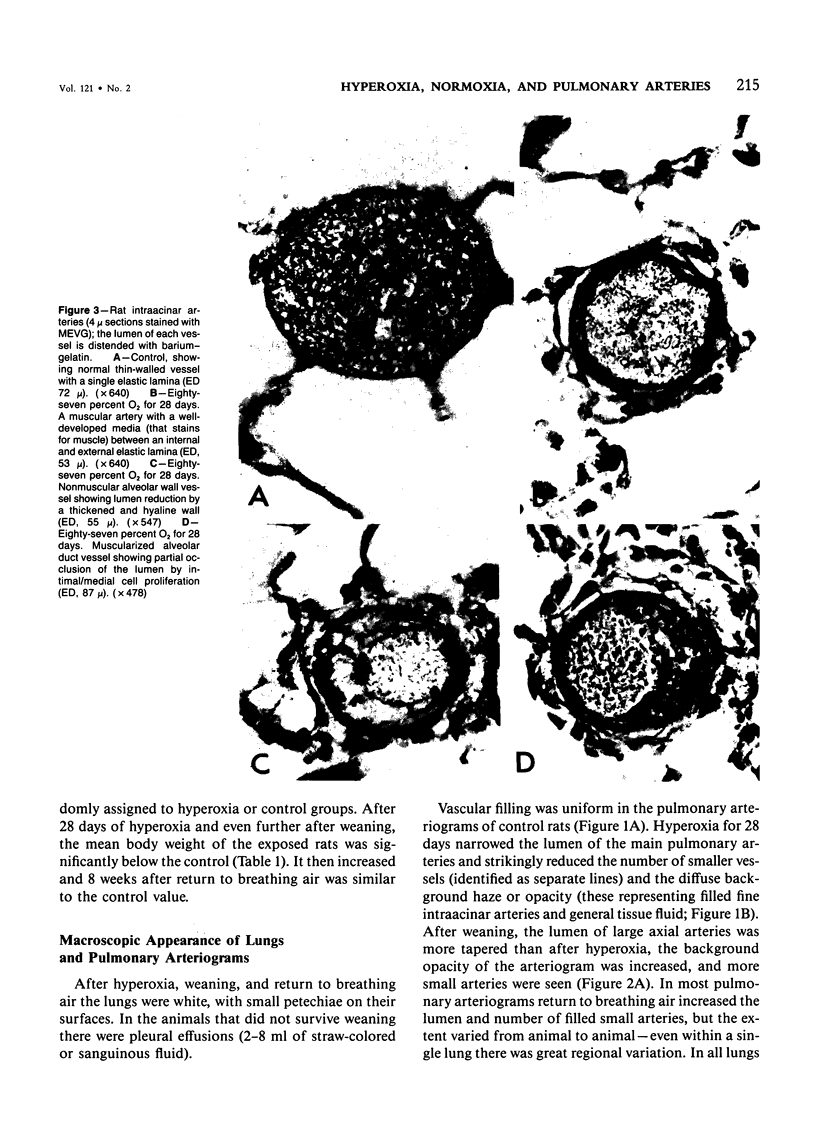
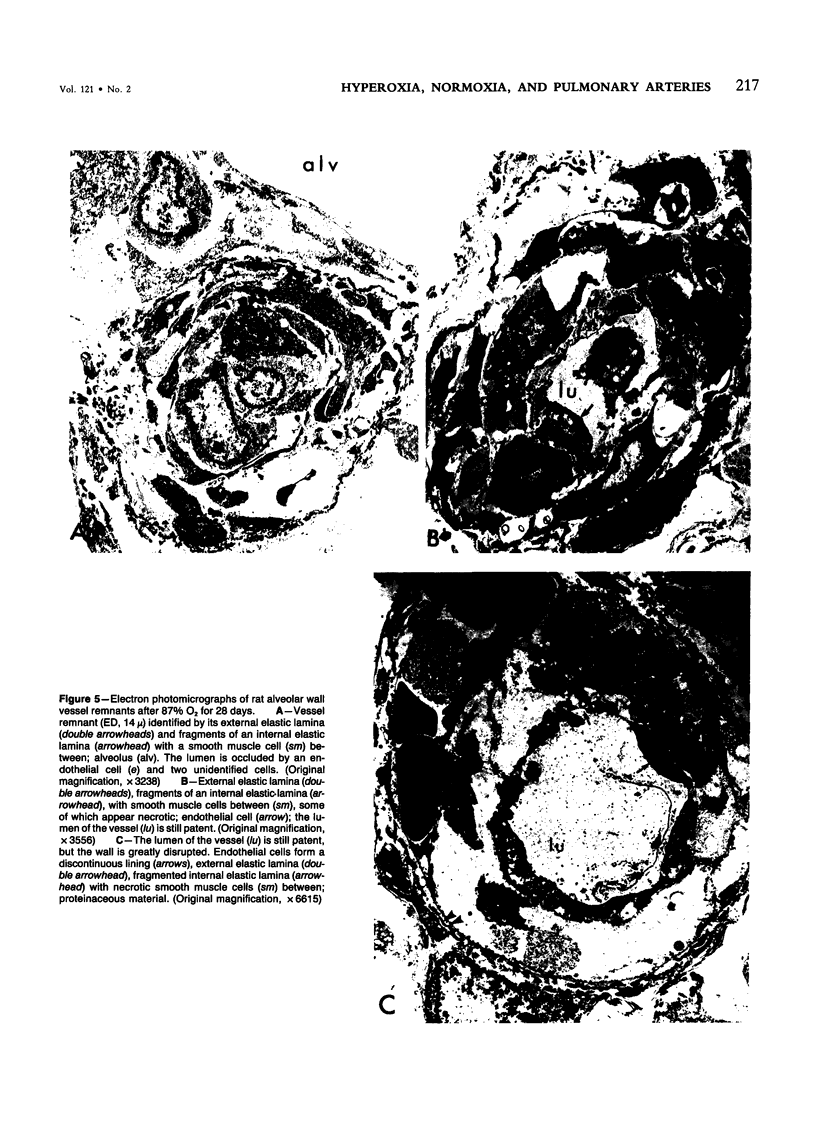
This study describes the pulmonary vascular lesions in rat pulmonary arteries and altered right ventricular weight after 1) prolonged exposure to hyperoxia (87% O2 for 4 weeks) at ambient pressure, 2) weaning from hyperoxia to air over 7 days, and 3) return to breathing air for 2, 4, or 8 weeks. Hyperoxia for 28 days narrows the lumen of intraacinar and preacinar arteries, increasing the percent medial thickness (%MT) by reducing the external diameter and thickening medial muscle. The ratio of patent intraacinar arteries to alveoli is significantly reduced, and pulmonary vascular obstruction and obliteration is evident by electron microscopy. A higher proportion of intraacinar and preacinar arteries have muscle in their wall than in the normal lung: in alveolar wall and duct regions, the proportion of partially muscular and muscular intraacinar arteries increases at the expense of nonmuscular ones (for both regions P chi 2 less than or equal to 0.001); and in arteries associated with terminal bronchioli and bronchioli the proportion of muscular arteries increases at the expense of partially muscular ones (for both regions P chi 2 less than or equal to 0.001). Both after weaning and after return to breathing air lumen size increases; but, even after 8 weeks, the %MT remains significantly increased, and the ratio of intraacinar arteries to alveoli is less than normal. After weaning, the proportion of muscularized intraacinar and preacinar arteries is similar to that after hyperoxia. Two weeks after return to breathing air, the proportion of muscularized alveolar wall and duct arteries is greater (for both regions P chi 2 less than or equal to 0.001). Even 8 weeks after return to breathing air more arteries are muscularized than normal (for both alveolar wall and duct regions P chi 2 less than or equal to 0.001), and within the alveolar wall still more are muscularized than after hyperoxia (P chi 2 less than or equal to 0.001). Hyperoxia causes right ventricular hypertrophy, reducing the ratio of the weight of the left ventricle and septum to that of the right ventricle (P chi 2 less than or equal to 0.001). Weaning further increases the hypertrophy, the ratio being further reduced (P chi 2 less than or equal to 0.001, compared with both hyperoxia and control values). On return to breathing air the degree of hypertrophy is less, but it persists, and even after 8 weeks the ratio is still less than normal (P chi 2 less than or equal to 0.01).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H., Wyatt J. P. Oxygen poisoning in mice. Ultrastructural and surfactant studies during exposure and recovery. Arch Pathol. 1970 Nov;90(5):463–472. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Endothelial regeneration as a marker of the differential vascular responses in oxygen-induced pulmonary edema. Lab Invest. 1974 Mar;30(3):350–357. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y., Wyatt J. P. Reaction of the lung cells to a high concentration of oxygen. Arch Pathol. 1968 Dec;86(6):671–675. [PubMed] [Google Scholar]
- Bowden D. H., Adamson Y. R. Reparative changes following pulmonary cell injury. Ultrastructural, cytodynamic, and surfactant studies in mice after oxygen exposure. Arch Pathol. 1971 Oct;92(4):279–283. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- FULTON R. M., HUTCHINSON E. C., JONES A. M. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952 Jul;14(3):413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R., Reid L. M. Early recovery from hypoxic pulmonary hypertension: a structural and functional study. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):1247–1253. doi: 10.1152/jappl.1984.57.4.1247. [DOI] [PubMed] [Google Scholar]
- Harrison G. A. Ultrastructural changes in rat lung during long-term exposure to oxygen. Exp Med Surg. 1971;29(1):96–107. [PubMed] [Google Scholar]
- Jones R., Zapol W. M., Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am J Pathol. 1984 Nov;117(2):273–285. [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kaplan H. P., Robinson F. R., Kapanci Y., Weibel E. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Lab Invest. 1969 Jan;20(1):94–100. [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Miller P. J. An elastin stain. Med Lab Technol. 1971 Apr;28(2):148–149. [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner F., Felig P., Trachtenberg E. Structure of rat lung after protracted oxygen breathing. Arch Pathol. 1967 Jan;83(1):99–107. [PubMed] [Google Scholar]
- Sjostrom K., Crapo J. D. Structural and biochemical adaptive changes in rat lungs after exposure to hypoxia. Lab Invest. 1983 Jan;48(1):68–79. [PubMed] [Google Scholar]
- Välimäki M., Niinikoski J. Development and reversibility of pulmonary oxygen poisoning in the rat. Aerosp Med. 1973 May;44(5):533–538. [PubMed] [Google Scholar]