Abstract
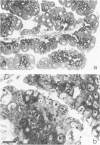
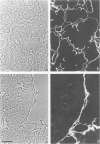
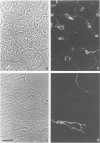
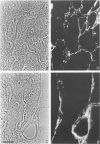
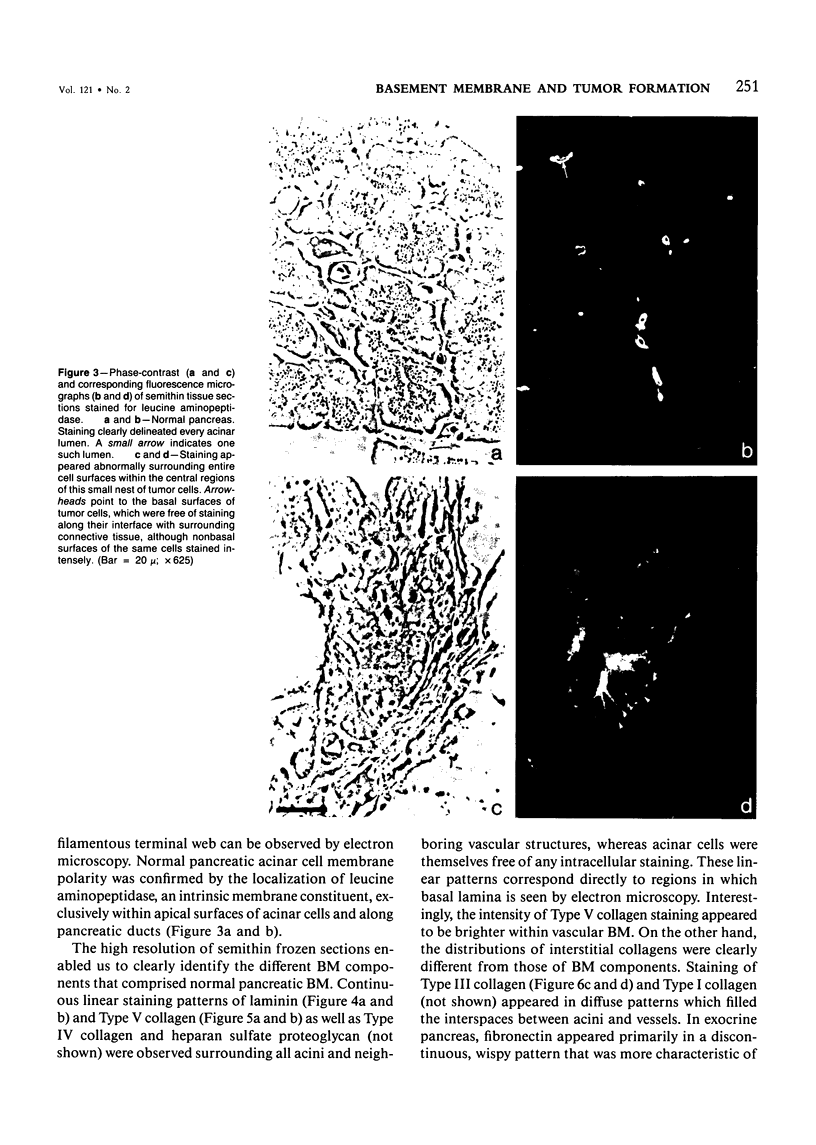
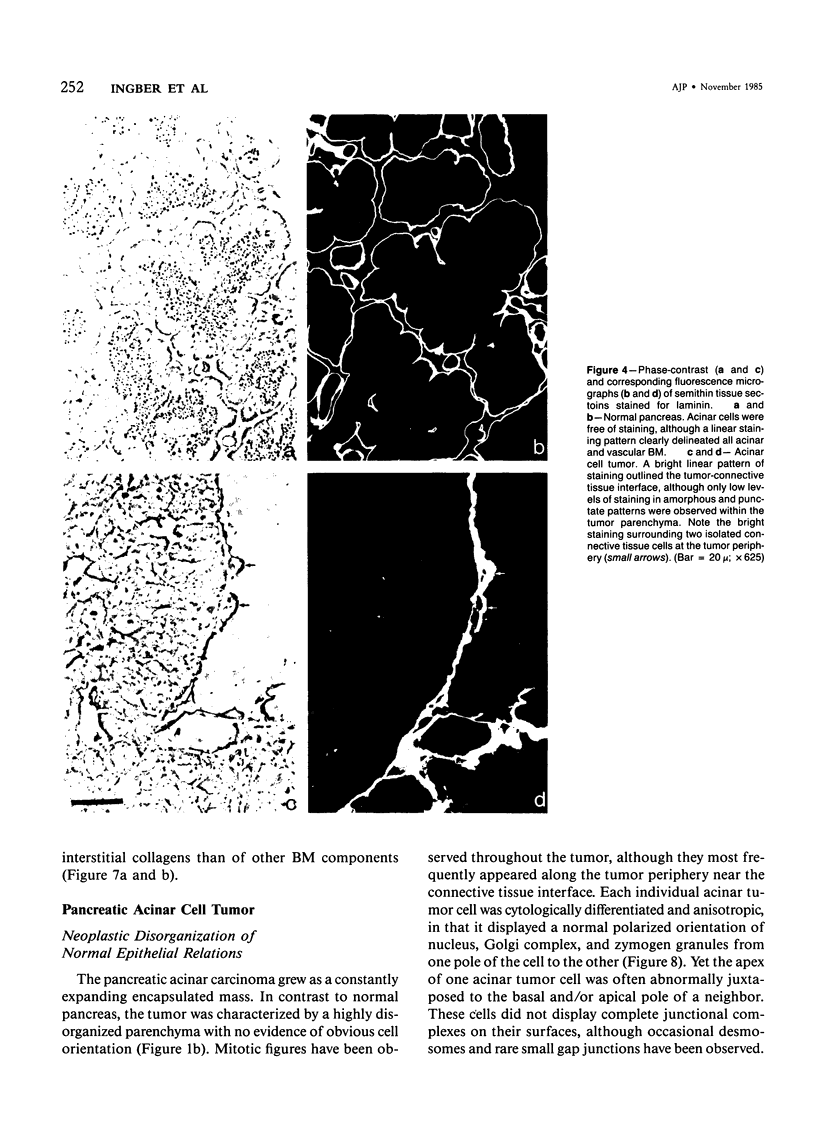
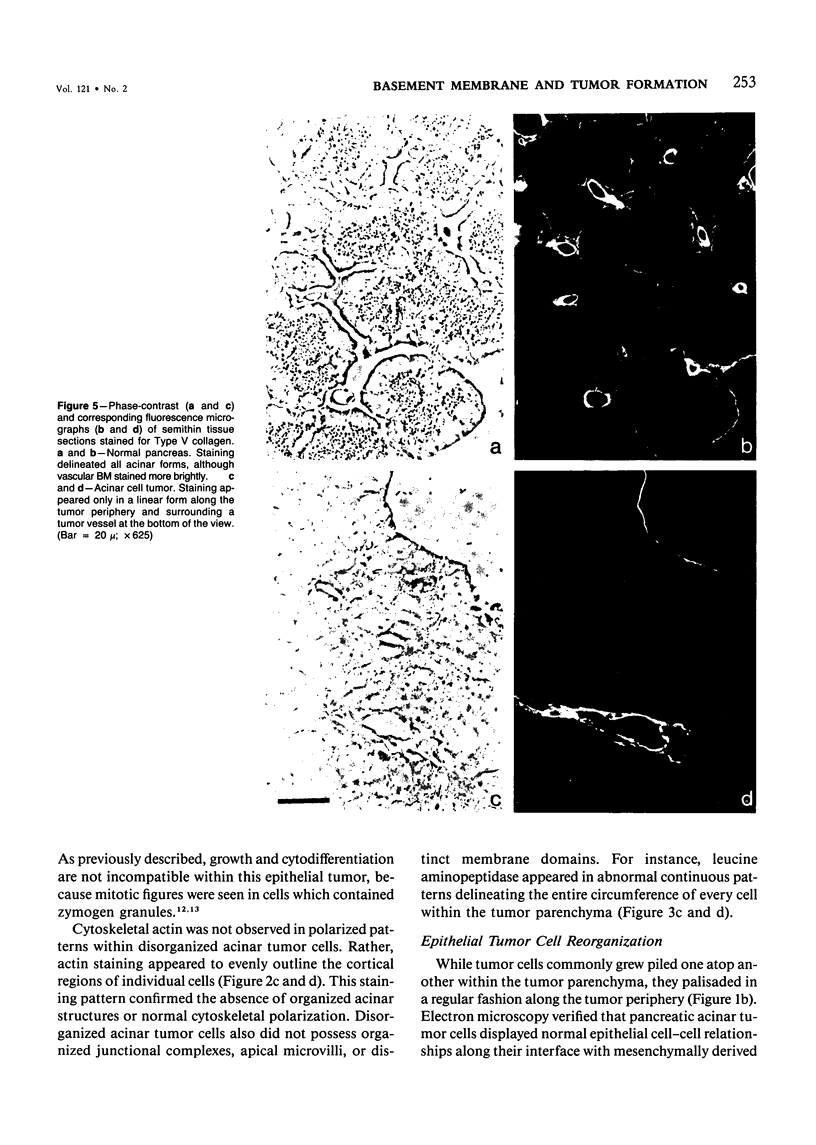
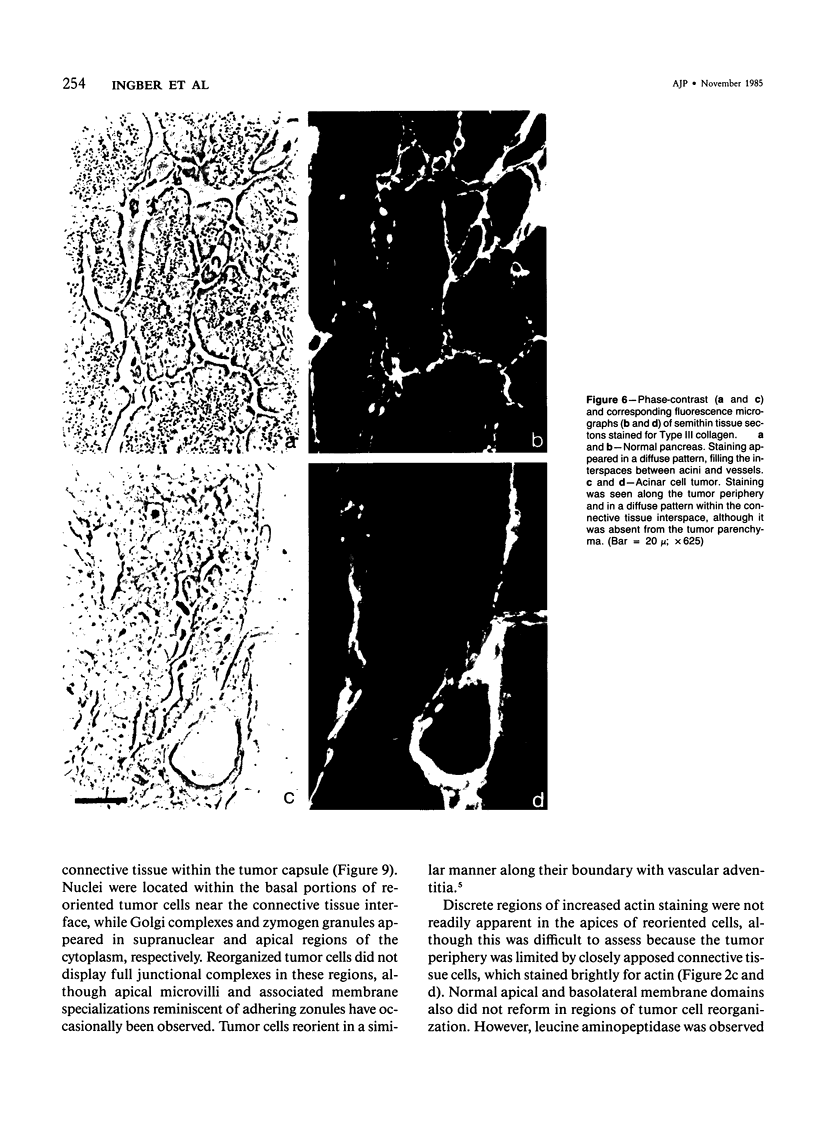
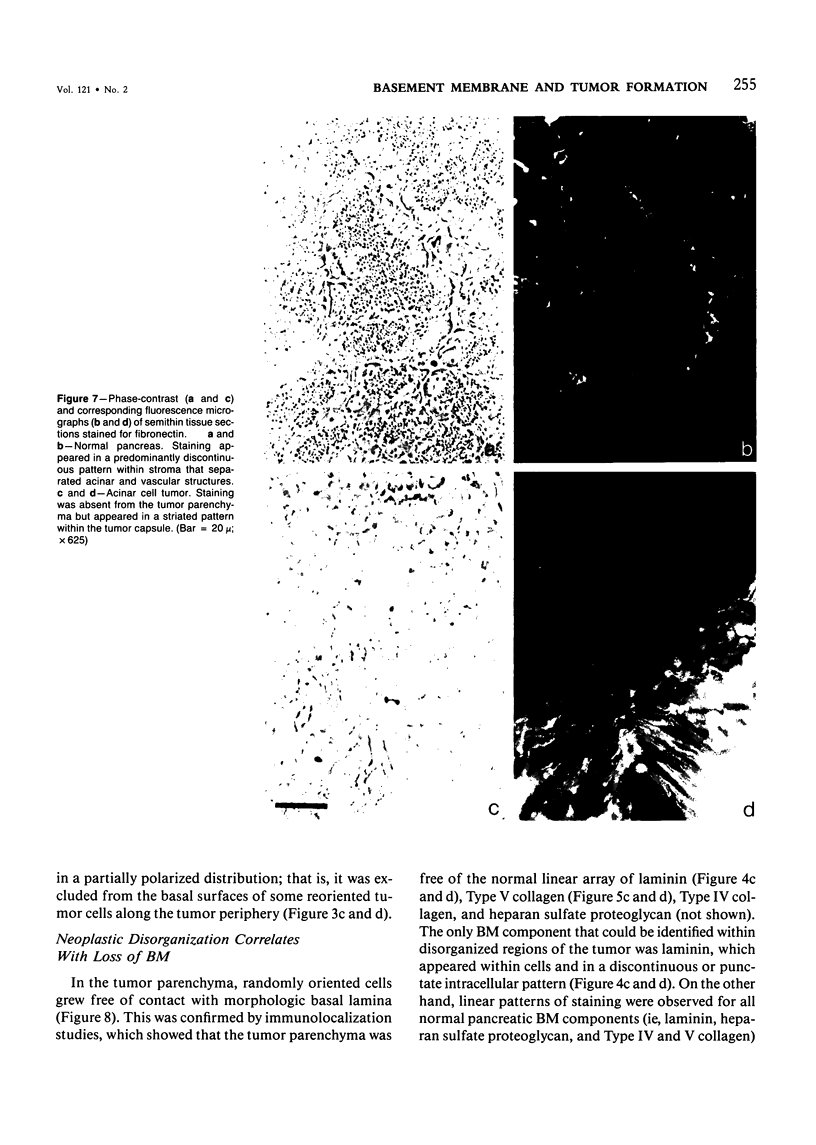
The authors have analyzed the structural relations of a nonmetastatic rat pancreatic acinar carcinoma and contrasted them with those of normal exocrine pancreas in order to better define the role of basement membrane (BM) in early stages of neoplastic disorganization. These studies showed that normal acinar cells rested on continuous BM (containing laminin, heparan sulfate proteoglycan, and Type IV and V collagens) and displayed a polarized distribution of intracellular organelles, cytoskeletal assemblies (concentration of actin within terminal web), and distinct membrane domains (apical leucine aminopeptidase). In contrast, the parenchyma of the pancreatic acinar carcinoma was free of all BM components except for a discontinuous array of laminin. In these regions, acinar tumor cells appeared randomly oriented, displayed actin in uniform cortical distributions, and lost membrane polarity. However, when tumor cells contacted mesenchymally derived connective tissue along tumor capsule and vascular adventitia, they accumulated intact BM and reoriented in a manner reminiscent of normal pancreas. Tumor cell reorganization was observed in the absence of formation of full junctional complexes or normally polarized membrane domains, although leucine aminopeptidase appeared to be excluded from regions of tumor cell surfaces that were in direct contact with BM. The loss of normal epithelial cell-cell arrangements that is the hallmark of early stages of tumor formation could therefore result from failure to match increases in cell number with commensurate BM extension.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Fambrough D. M. Aggregates of acetylcholine receptors are associated with plaques of a basal lamina heparan sulfate proteoglycan on the surface of skeletal muscle fibers. J Cell Biol. 1983 Nov;97(5 Pt 1):1396–1411. doi: 10.1083/jcb.97.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becich M. J., Bendayan M., Reddy J. K. Intracellular transport and storage of secretory proteins in relation to cytodifferentiation in neoplastic pancreatic acinar cells. J Cell Biol. 1983 Apr;96(4):949–960. doi: 10.1083/jcb.96.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell A. G., Bessem C. C., Slavkin H. C. Possible functions of mesenchyme cell-derived fibronectin during formation of basal lamina. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3711–3715. doi: 10.1073/pnas.78.6.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Sekkingstad M., Meloy B. A. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit and human tissues. Differentiation. 1983;24(2):174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Cutler L. S., Chaudhry A. P. Intercellular contacts at the epithelial-mesenchymal interface during the prenatal development of the rat submandibular gland. Dev Biol. 1973 Aug;33(2):229–240. doi: 10.1016/0012-1606(73)90133-4. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe C. J., Morgan W. D., Slatick M. S. Influence of epithelio-mesenchymal interactions on tumor induction by polyoma virus. Int J Cancer. 1966 Sep 15;1(5):419–450. doi: 10.1002/ijc.2910010504. [DOI] [PubMed] [Google Scholar]
- DeCosse J. J., Gossens C. L., Kuzma J. F., Unsworth B. R. Breast cancer: induction of differentiation by embryonic tissue. Science. 1973 Sep 14;181(4104):1057–1058. doi: 10.1126/science.181.4104.1057. [DOI] [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagenous stroma by isolated epithelium grown in vitro. Exp Cell Res. 1971 Mar;65(1):215–220. doi: 10.1016/s0014-4827(71)80069-1. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Alitalo K., Vaheri A., Timpl R., Saxén L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):485–489. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Form D. M., VanDeWater L., Dvorak H. F., Smith B. D. Pathogenesis of tumor desmoplasia. II. Collagens synthesized by line 1 and line 10 guinea pig carcinoma cells and by syngeneic fibroblasts in vitro. J Natl Cancer Inst. 1984 Nov;73(5):1207–1214. [PubMed] [Google Scholar]
- Fujii H., Cunha G. R., Norman J. T. The induction of adenocarcinomatous differentiation in neoplastic bladder epithelium by an embryonic prostatic inductor. J Urol. 1982 Oct;128(4):858–861. doi: 10.1016/s0022-5347(17)53221-8. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Grover A., Andrews G., Adamson E. D. Role of laminin in epithelium formation by F9 aggregates. J Cell Biol. 1983 Jul;97(1):137–144. doi: 10.1083/jcb.97.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Tilney L. G., Fujiwara K., Heuser J. E. Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J Cell Biol. 1982 Aug;94(2):425–443. doi: 10.1083/jcb.94.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. The proteins of oncogenes. Sci Am. 1984 Aug;251(2):70–79. doi: 10.1038/scientificamerican0884-70. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Jamieson J. D. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanij V., Jamieson J. D. Comparison of secretory protein profiles in developing rat pancreatic rudiments and rat acinar tumor cells. J Cell Biol. 1982 Dec;95(3):742–746. doi: 10.1083/jcb.95.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanij V., Jamieson J. D. Comparison of secretory protein profiles in developing rat pancreatic rudiments and rat acinar tumor cells. J Cell Biol. 1982 Dec;95(3):742–746. doi: 10.1083/jcb.95.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I. Structure and composition of early basement membranes: studies with early embryos and teratocarcinoma cells. Med Biol. 1983 Feb;61(1):1–30. [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980 Jul;11(4):353–366. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Castiglioni G., Parma R., Nassivera N., De Camilli P. Ca++-dependent disassembly and reassembly of occluding junctions in guinea pig pancreatic acinar cells. Effect of drugs. J Cell Biol. 1978 Oct;79(1):156–172. doi: 10.1083/jcb.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza I., Ibarra G., Sabanero M., Martínez-Palomo A., Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet R. L., Clark W. R., Williams R. H., Rutter W. J. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972 Dec;29(4):436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Reddy J. K. Transplantable acinar cell carcinoma of the rat pancreas. Am J Pathol. 1979 Feb;94(2):333–348. [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S. Transplantable pancreatic carcinoma of the rat. Science. 1977 Oct 7;198(4312):78–80. doi: 10.1126/science.897688. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S., Warren J. R., Qureshi S. A., Christensen E. I. Differentiation and DNA synthesis in pancreatic acinar carcinoma of rat. Cancer Res. 1980 Oct;40(10):3443–3454. [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M. Basement membrane polarizes lectin binding sites of Drosophila larval fat body cells. Nature. 1983 May 26;303(5915):340–342. doi: 10.1038/303340a0. [DOI] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Albert J., Furthmayr H. Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. an immunoferritin study of ultrathin frozen sections. J Cell Biol. 1980 Jun;85(3):597–616. doi: 10.1083/jcb.85.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Furthmayr H. A new method of iodinating collagens for use in radioimmunoassay. Anal Biochem. 1979 Jul 15;96(2):489–499. doi: 10.1016/0003-2697(79)90611-0. [DOI] [PubMed] [Google Scholar]
- Sakakura T., Nishizuka Y., Dawe C. J. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976 Dec 24;194(4272):1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Bernfield M. Mesenchyme cells degrade epithelial basal lamina glycosaminoglycan. Dev Biol. 1982 Dec;94(2):378–390. doi: 10.1016/0012-1606(82)90355-4. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Cohen H. I., Faubion J. Development of the embryonic mammalian pancreas: the relationship between morphogenesis and cytodifferentiation. Dev Biol. 1977 Dec;61(2):119–130. doi: 10.1016/0012-1606(77)90285-8. [DOI] [PubMed] [Google Scholar]
- U H. S., Evans-Layng M. Polar redistribution of Na+K+ATPase in aggregating MDCK cells. Exp Cell Res. 1983 Jun;146(1):192–198. doi: 10.1016/0014-4827(83)90337-3. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Christian C. N., Vigny M., Bauer H. C., Sonderegger P., Daniels M. P. Laminin induces acetylcholine receptor aggregation on cultured myotubes and enhances the receptor aggregation activity of a neuronal factor. J Neurosci. 1983 May;3(5):1058–1068. doi: 10.1523/JNEUROSCI.03-05-01058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- WESSELLS N. K. DNA SYNTHESIS, MITOSIS, AND DIFFERENTIATION IN PANCREATIC ACINAR CELLS IN VITRO. J Cell Biol. 1964 Mar;20:415–433. doi: 10.1083/jcb.20.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. K., Hansen L. J., Reddy N. K., Kanwar Y. S., Reddy J. K. Differentiation of pancreatic acinar carcinoma cells cultured on rat testicular seminiferous tubular basement membranes. Cancer Res. 1984 Nov;44(11):5361–5368. [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Lewko W. M., Kidwell W. R. Blocking basement membrane collagen deposition inhibits the growth of 7,12-dimethylbenzanthracene-induced rat mammary tumors. Cancer Lett. 1981 Mar;12(1-2):9–21. doi: 10.1016/0304-3835(81)90032-x. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Vonderhaar B. K., Kidwell W. R. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980 Dec;80(2):253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]