Abstract
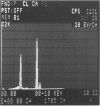
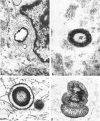
Calcific degeneration is the main cause of porcine bioprosthetic valve failure. This dystrophic phenomenon has been studied by transmission electron microscopy in 26 explants; six normally processed unimplanted xenografts and a pig aortic valve from the slaughterhouse served as controls. Loss of endothelial lining and proteoglycans were a regular finding in all the commercially processed valves. In order to detect initial calcifications, we investigated in particular areas apparently devoid of mineralization at x-ray. Three main ultramicroscopic features were found: 1) intracytoplasmic and interstitial calcospherulae in 22 explants, 2) calcified collagen fibrils in 15, and 3) platelike calcium deposits upon amorphous material in 3. X-ray diffraction and energy-dispersive microanalysis identified Ca2+ deposits as crystals of hydroxyapatite. From these findings there is evidence that debris and membrane fragments of the pig cusp cells represent one of the initial nuclei of calcification.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C., Matsuzawa T., Sajdera S. W., Ali S. Y. Membranous particles in calcifying cartilage matrix. Trans N Y Acad Sci. 1970 May;32(5):619–630. doi: 10.1111/j.2164-0947.1970.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Anderson H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969 Apr;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbustini E., Bortolotti U., Valente M., Milano A., Gallucci V., Pennelli N., Thiene G. Cusp disruption by massive lipid infiltration. A rare cause of porcine valve dysfunction. J Thorac Cardiovasc Surg. 1982 Nov;84(5):738–743. [PubMed] [Google Scholar]
- Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967 Sep;20(1):33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- Bortolotti U., Milano A., Mazzucco A., Valfre C., Fasoli G., Valente M., Thiene G., Gallucci V. Longevity of the formaldehyde-preserved Hancock porcine heterograft. J Thorac Cardiovasc Surg. 1982 Sep;84(3):451–453. [PubMed] [Google Scholar]
- Cipriano P. R., Billingham M. E., Oyer P. E., Kutsche L. M., Stinson E. B. Calcification of porcine prosthetic heart valves: a radiographic and light microscopy study. Circulation. 1982 Nov;66(5):1100–1104. doi: 10.1161/01.cir.66.5.1100. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J., Boyce S. W., Billingham M. E., Jones M., Ishihara T., Roberts W. C. Calcific deposits in porcine bioprostheses: structure and pathogenesis. Am J Cardiol. 1980 Nov;46(5):721–734. doi: 10.1016/0002-9149(80)90421-x. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Levy R. J., Ferrans V. J., Dearden L. C., Nashef A., Goodman A. P., Carpentier A. Calcifications of cardiac valve bioprostheses. Biochemical, histologic, and ultrastructural observations in a subcutaneous implantation model system. J Thorac Cardiovasc Surg. 1982 Apr;83(4):602–609. [PubMed] [Google Scholar]
- Kim K. M. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. 1976 Feb;35(2):156–162. [PubMed] [Google Scholar]
- Levy R. J., Schoen F. J., Howard S. L. Mechanism of calcification of porcine bioprosthetic aortic valve cusps: role of T-lymphocytes. Am J Cardiol. 1983 Sep 1;52(5):629–631. doi: 10.1016/0002-9149(83)90040-1. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Schoen F. J., Levy J. T., Nelson A. C., Howard S. L., Oshry L. J. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. 1983 Nov;113(2):143–155. [PMC free article] [PubMed] [Google Scholar]
- Levy R. J., Zenker J. A., Bernhard W. F. Porcine bioprosthetic valve calcification in bovine left ventricle-aorta shunts: studies of the deposition of vitamin K-dependent proteins. Ann Thorac Surg. 1983 Aug;36(2):187–192. doi: 10.1016/s0003-4975(10)60454-7. [DOI] [PubMed] [Google Scholar]
- Milano A., Bortolotti U., Talenti E., Valfrè C., Arbustini E., Valente M., Mazzucco A., Gallucci V., Thiene G. Calcific degeneration as the main cause of porcine bioprosthetic valve failure. Am J Cardiol. 1984 Apr 1;53(8):1066–1070. doi: 10.1016/0002-9149(84)90638-6. [DOI] [PubMed] [Google Scholar]
- Price P. A., Poser J. W., Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3374–3375. doi: 10.1073/pnas.73.10.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray T. L., Roberts W. C. Structural changes in porcine xenografts used as substitute cardiac valves. Gross and histologic observations in 51 glutaraldehyde-preserved Hancock valves in 41 patients. Am J Cardiol. 1977 Sep;40(3):319–330. doi: 10.1016/0002-9149(77)90153-9. [DOI] [PubMed] [Google Scholar]
- Thubrikar M. J., Deck J. D., Aouad J., Nolan S. P. Role of mechanical stress in calcification of aortic bioprosthetic valves. J Thorac Cardiovasc Surg. 1983 Jul;86(1):115–125. [PubMed] [Google Scholar]
- Valente M., Arbustini E., Bortolotti U., Talenti E., Thiene G. Perforation of muscle shelf of right coronary cusp causing acute regurgitation of porcine mitral xenograft. Am Heart J. 1984 Jul;108(1):180–183. doi: 10.1016/0002-8703(84)90569-6. [DOI] [PubMed] [Google Scholar]
- Valente M., Bortolotti U., Arbustini E., Talenti E., Thiene G., Gallucci V. Glutaraldehyde-preserved porcine bioprosthesis. Factors affecting performance as determined by pathologic studies. Chest. 1983 Apr;83(4):607–611. doi: 10.1378/chest.83.4.607. [DOI] [PubMed] [Google Scholar]