Abstract
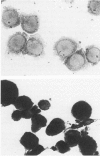
The tumor-promoting ester 4 beta-phorbol 12-myristate 13-acetate (PMA) has been shown to induce the differentiation of the immature monocytelike cell line U-937 c in vitro into a heterogeneous population of cells, including small "dense" cells, large vacuolized or "foamy" cells, spindle-shaped cells, and cells with multiple filopodia ("stellate" cells). The effect of PMA was dose- and time-dependent, the optimal conditions being 40-162 nM PMA for 48 hours. The minimum time of exposure to PMA to ensure further differentiation of U-937 cells was about 5 hours. The PMA-stimulated cells acquired morphologic, ultrastructural, and functional characteristics typical of cells of the monocyte/macrophage lineage. The PMA-treated U-937 cells became adherent, ceased to proliferate, and exhibited increased expression of monocyte-specific antigens (Leu-M2, - M3, HLADr), surface receptors (FcR, C3bR), enzymes (nonspecific esterase, transglutaminase), and ability to mediate chemotaxis, phagocytosis, superoxide anion production, and antibody-dependent cytotoxicity reactions. The induced cells lost their morphologic differentiation and ability to attach to surfaces and regained proliferative capacity upon repeated subculture in PMA-free media.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Unanue E. R. Regulation of macrophage populations. II. Synthesis and expression of Ia antigens by peritoneal exudate macrophages is a transient event. J Immunol. 1981 Jan;126(1):263–269. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Baird W. M. Tumor promoters and the mechanism of tumor promotion. Adv Cancer Res. 1980;32:1–74. doi: 10.1016/s0065-230x(08)60360-7. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Dunn P. A., Tyrer H. W. Quantitation of neutrophil phagocytosis, using fluorescent latex beads. Correlation of microscopy and flow cytometry. J Lab Clin Med. 1981 Sep;98(3):374–381. [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fibach E., Yamasaki H., Weinstein I. B., Marks P. A., Rifkind R. A. Heterogeneity of murine erythroleukemia cells with respect to tumor promoter-mediated inhibition of cell differentiation. Cancer Res. 1978 Nov;38(11 Pt 1):3685–3688. [PubMed] [Google Scholar]
- Goodwin B. J., Weinberg J. B. Receptor-mediated modulation of human monocyte, neutrophil, lymphocyte, and platelet function by phorbol diesters. J Clin Invest. 1982 Oct;70(4):699–706. doi: 10.1172/JCI110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet F. C., Charron D. J., Fendly B. M., Levy R., Ness D. B. HLA-DR epitope region definition by use of monoclonal antibody probes. J Immunol. 1980 Dec;125(6):2785–2789. [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Hattori T., Pack M., Bougnoux P., Chang Z. L., Hoffman T. Interferon-induced differentiation of U937 cells. Comparison with other agents that promote differentiation of human myeloid or monocytelike cell lines. J Clin Invest. 1983 Jul;72(1):237–244. doi: 10.1172/JCI110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Wenner C. E., Kemp G., Papahadjopoulos D. Surface properties of phorbol esters and their interaction with lipid monolayers and bilayers. Cancer Res. 1975 Nov;35(11 Pt 1):2991–2995. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phorbol myristate acetate stimulates phagosome-lysosome fusion in mouse macrophages. J Exp Med. 1981 Jul 1;154(1):101–111. doi: 10.1084/jem.154.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Moore J. O., Metzgar R. S. Preparation and characterization of lymphocyte-activating factor (LAF) from acute monocytic and myelomonocytic leukemia cells. Cell Immunol. 1978 Nov;41(1):199–206. doi: 10.1016/s0008-8749(78)80040-9. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Fischer D. G., Anderson S. J., Koren H. S. Characterization of a human macrophage-like cell line stimulated in vitro: a model of macrophage functions. J Immunol. 1980 Jul;125(1):6–12. [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman G. H., Preisler H. D., Papahadjopoulos D. Membrane action of DMSO and other chemical inducers of Friend leukaemic cell differentiation. Nature. 1976 Jul 29;262(5567):361–363. doi: 10.1038/262360a0. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Andersson L. c., Gahmberg C. G. Cell surface characteristics of human histiocytic lymhoma lines--I. Surface glycoprotein patterns. Leuk Res. 1980;4(3):271–277. doi: 10.1016/0145-2126(80)90034-x. [DOI] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Fischer D. G., Koren H. S., Snyderman R. Development of specific receptors for N-formylated chemotactic peptides in a human monocyte cell line stimulated with lymphokines. J Exp Med. 1980 Jul 1;152(1):31–40. doi: 10.1084/jem.152.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler H. D., Christoff G., Taylor E. Cryoprotective agents as inducers of erythroleukemic cell differentiation in vitro. Blood. 1976 Mar;47(3):363–368. [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroff G., Neumann C., Sorg C. Transglutaminase as a marker for subsets of murine macrophages. Eur J Immunol. 1981 Aug;11(8):637–642. doi: 10.1002/eji.1830110809. [DOI] [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Phenotypic expression of transformation: induction in cell culture by a phorbol ester. Science. 1967 Sep 22;157(3795):1443–1444. doi: 10.1126/science.157.3795.1443. [DOI] [PubMed] [Google Scholar]
- Stuart R. K., Hamilton J. A. Tumor-promoting phorbol esters stimulate hematopoietic colony formation in vitro. Science. 1980 Apr 25;208(4442):402–404. doi: 10.1126/science.6245446. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Lee L. S., Fisher P. B., Mufson A., Yamasaki H. Action of phorbol esters in cell culture: mimicry of transformation, altered differentiation, and effects on cell membranes. J Supramol Struct. 1979;12(2):195–208. doi: 10.1002/jss.400120206. [DOI] [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]