Abstract
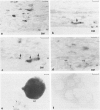
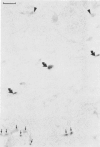
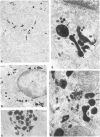
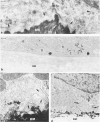
Positive identification of osteoclast percursors has not yet been possible. The authors have, in the present report, used a model system in the rat in which it is possible to induce the formation of multinucleated osteoclasts at a predictable and reproducible site and time (Tran Van P, Vignery A, Baron R. Anat Rec 1982, 202:445-451; Cell Tissue Res 1982, 225:283-292). This system allowed the investigation of the cellular events occurring locally during the recruitment and differentiation of osteoclast precursors. Prior to the formation of multinucleated osteoclasts, mononuclear cells positive for fluoride-inhibitable nonspecific esterase and cells positive for tartrate-resistant acid phosphatase increase in number locally. Double staining procedures demonstrated the presence of both enzymes in a number of cells, thereby suggesting that they are steps in the differentiation of a single cell population. Ultrastructural studies show that lysosomal enzymes are present in every compartment of the biosynthetic pathway, in small primary lysosomes and various forms of storage granules. As these precursors arrive at the bone surface, the storage granule lysosomes are markedly depleted. It is concluded that mononuclear precursors of the osteoclast are members of the mononuclear-phagocyte lineage and differentiate early to synthesize, store, and later secrete large quantities of lysosomal enzymes. The mature osteoclast, which, as its precursor, is positive for the mononuclear-phagocyte marker enzyme nonspecific esterase, results from the fusion of these mononuclear precursors, which occurs only after their attachment to the bone surface to be resorbed.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J Cell Biol. 1982 Sep;94(3):613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash P., Loutit J. F., Townsend K. M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980 Feb 14;283(5748):669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Origin of granules in polymorphonuclear leukocytes. Two types derived from opposite faces of the Golgi complex in developing granulocytes. J Cell Biol. 1966 Feb;28(2):277–301. doi: 10.1083/jcb.28.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970 Apr;45(1):54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. R., Golde D. W. Differentiation of macrophages from normal human bone marrow in liquid culture. Electron microscopy and cytochemistry. J Clin Invest. 1978 Jun;61(6):1555–1569. doi: 10.1172/JCI109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentfeld-Barker M. E., Bainton D. F. Cytochemical localization of arylsulfatase B in rat basophils and mast cells. J Histochem Cytochem. 1980 Oct;28(10):1055–1061. doi: 10.1177/28.10.7419898. [DOI] [PubMed] [Google Scholar]
- Bozdech M. J., Bainton D. F. Identification of alpha-naphthyl butyrate esterase as a plasma membrane ectoenzyme of monocytes and as a discrete intracellular membrane-bounded organelle in lymphocytes. J Exp Med. 1981 Jan 1;153(1):182–195. doi: 10.1084/jem.153.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E. H., Van der Meer J. W., van de Gevel J. S., Gribnau J. C., Thesingh G. W., van Furth R. In vitro formation of osteoclasts from long-term cultures of bone marrow mononuclear phagocytes. J Exp Med. 1982 Dec 1;156(6):1604–1614. doi: 10.1084/jem.156.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger E. H., van der Meer J. W., Nijweide P. J. Osteoclast formation from mononuclear phagocytes: role of bone-forming cells. J Cell Biol. 1984 Dec;99(6):1901–1906. doi: 10.1083/jcb.99.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Doty S. B., Schofield B. H. Electron microscopic localization of hydrolytic enzymes in osteoclasts. Histochem J. 1972 May;4(3):245–258. doi: 10.1007/BF01890996. [DOI] [PubMed] [Google Scholar]
- Ejiri S. The preosteoclast and its cytodifferentiation into the osteoclast: ultrastructural and histochemical studies of rat fetal parietal bone. Arch Histol Jpn. 1983 Sep;46(4):533–557. [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Gay C. V., Ito M. B., Schraer H. Carbonic anhydrase activity in isolated osteoclasts. Metab Bone Dis Relat Res. 1983;5(1):33–39. doi: 10.1016/0221-8747(83)90048-6. [DOI] [PubMed] [Google Scholar]
- Goldfischer S. The cytochemical demonstration of lysosomal aryl sulfatase activity by light and electron microscopy. J Histochem Cytochem. 1965 Jul-Aug;13(6):520–523. doi: 10.1177/13.6.520. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gordon S., Cohn Z. A. The macrophage. Int Rev Cytol. 1973;36:171–214. doi: 10.1016/s0074-7696(08)60218-1. [DOI] [PubMed] [Google Scholar]
- Gordon S. Macrophage neutral proteinases and chronic inflammation. Ann N Y Acad Sci. 1976;278:176–189. doi: 10.1111/j.1749-6632.1976.tb47028.x. [DOI] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. Fine structural localization of acid phosphomonoesterase in the brush border region of osteoclasts. Histochemie. 1971;28(4):337–344. doi: 10.1007/BF00702639. [DOI] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. On the histogenesis of the cells in fracture callus. Electron microscopic autoradiographic observations in parabiotic rats and studies on labeled monocytes. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):318–329. [PubMed] [Google Scholar]
- Heyden G. The effect of sodium ethylenediaminetetra-acetate (Na2 EDTA) on the histochemical demonstration of some enzyme activities in neonatal mouse molar tissues. Histochemie. 1969;20(2):171–180. doi: 10.1007/BF00268711. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Rimmer E. F., Moore A., Chambers T. J. On the origin of the osteoclast: the cell surface phenotype of rodent osteoclasts. Calcif Tissue Int. 1985 Jan;37(1):46–50. doi: 10.1007/BF02557678. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Roodman G. D., McManus L. M., Mundy G. R. Identification and characterization of osteoclast-like cells and their progenitors in cultures of feline marrow mononuclear cells. J Cell Biol. 1984 Aug;99(2):471–480. doi: 10.1083/jcb.99.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEE W. S., NOLAN P. D. ORIGIN OF OSTEOCLASTS FROM THE FUSION OF PHAGOCYTES. Nature. 1963 Oct 19;200:225–226. doi: 10.1038/200225a0. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Simmons D. J. Investigation of cell lineage in bone using a chimaera of chick and quial embryonic tissue. Nature. 1975 Nov 27;258(5533):325–327. doi: 10.1038/258325a0. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Simmons D. J., Krukowski M. Osteoclast precursor cells are present in the blood of preossification chick embryos. Dev Biol. 1981 May;84(1):230–234. doi: 10.1016/0012-1606(81)90388-2. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Ko J. S., Bernard G. W. Osteoclast formation in vitro from bone marrow mononuclear cells in osteoclast-free bone. Am J Anat. 1981 Aug;161(4):415–425. doi: 10.1002/aja.1001610407. [DOI] [PubMed] [Google Scholar]
- Leis J. F., Kaplan N. O. An acid phosphatase in the plasma membranes of human astrocytoma showing marked specificity toward phosphotyrosine protein. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6507–6511. doi: 10.1073/pnas.79.21.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Lucht U. Acid phosphatase of osteoclasts demonstrated by electron microscopic histochemistry. Histochemie. 1971;28(2):103–117. doi: 10.1007/BF00279855. [DOI] [PubMed] [Google Scholar]
- Luk S. C., Nopajaroonsri C., Simon G. T. The ultrastructure of cortical bone in young adult rabbits. J Ultrastruct Res. 1974 Feb;46(2):184–205. doi: 10.1016/s0022-5320(74)80055-9. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Walker D. G. The hematogenous origin of osteoclasts: experimental evidence from osteopetrotic (microphthalmic) mice treated with spleen cells from beige mouse donors. Am J Anat. 1981 May;161(1):1–10. doi: 10.1002/aja.1001610102. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982 May;34(3):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Nelson R. L., Bauer G. E. Isolation of osteoclasts by velocity sedimentation at unit gravity. Calcif Tissue Res. 1977 Feb 11;22(3):303–313. doi: 10.1007/BF02010369. [DOI] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osdoby P., Martini M. C., Caplan A. I. Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J Exp Zool. 1982 Dec 30;224(3):331–344. doi: 10.1002/jez.1402240306. [DOI] [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R., Kulenkampff C., Staudinger M., Stein H. Lysosomal acid esterase: activity and isoenzymes in separated normal human blood cells. Blood. 1980 Jun;55(6):891–897. [PubMed] [Google Scholar]
- Ries W. L. Osteogenic periosteum esterase activity: a comparative morphological and cytochemical study of bone cells in situ on rat proximal tibiae and in smears. J Histochem Cytochem. 1984 Jan;32(1):55–62. doi: 10.1177/32.1.6197438. [DOI] [PubMed] [Google Scholar]
- Rifkin B. R., Brand J. S., Cushing J. E., Coleman S. J., Sanavi F. Fine structure of fetal rat calvarium; provisional identification of preosteoclasts. Calcif Tissue Int. 1980;31(1):21–28. doi: 10.1007/BF02407163. [DOI] [PubMed] [Google Scholar]
- Scott B. L. The occurrence of specific cytoplasmic granules in the osteoclast. J Ultrastruct Res. 1967 Aug 30;19(5):417–431. doi: 10.1016/s0022-5320(67)80071-6. [DOI] [PubMed] [Google Scholar]
- Scott B. L. Thymidine-3H electron microscope radioautography of osteogenic cells in the fetal rat. J Cell Biol. 1967 Oct;35(1):115–126. doi: 10.1083/jcb.35.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkler S. M., Linder J. E., Williams D. M., Johnson N. W. Formation of osteoclasts from blood monocytes during 1 alpha-OH Vit D-stimulated bone resorption in mice. J Anat. 1981 Oct;133(Pt 3):389–396. [PMC free article] [PubMed] [Google Scholar]
- Tran Van P. T., Vignery A., Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec. 1982 Apr;202(4):445–451. doi: 10.1002/ar.1092020403. [DOI] [PubMed] [Google Scholar]
- Tran Van P., Vignery A., Baron R. An electron-microscopic study of the bone-remodeling sequence in the rat. Cell Tissue Res. 1982;225(2):283–292. doi: 10.1007/BF00214682. [DOI] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975 Nov 21;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- Walker D. G. Control of bone resorption by hematopoietic tissue. The induction and reversal of congenital osteopetrosis in mice through use of bone marrow and splenic transplants. J Exp Med. 1975 Sep 1;142(3):651–663. doi: 10.1084/jem.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G. Spleen cells transmit osteopetrosis in mice. Science. 1975 Nov 21;190(4216):785–787. doi: 10.1126/science.1198094. [DOI] [PubMed] [Google Scholar]