Abstract
HPD is known to localize in neoplastic cells and when exposed to the appropriate wavelength of light causes cytotoxicity. The authors have established a rat urothelial cell model for use in comparing and contrasting the effects of HPD photodynamic therapy (PDT) in normal (RBL-01) and transitional cell carcinoma (AY27) bladder cell lines. Uptake, toxicity, and morphologic damage following exposure to HPD PDT were evaluated. Trypan blue exclusion was used for determination of the toxicity of several HPD concentrations (1, 10, 25, and 50 micrograms/ml) with increasing duration of incubation with HPD (0, 1, 2, 4, 12, 24, and 48 hours). Both cell lines displayed increased toxicity with higher concentrations of HPD; however, the AY27 cells were more susceptible to the toxic effects of HPD PDT than the RBL-01 cells at the higher HPD doses studied (25 and 50 micrograms/ml). Viability decreased with increased duration of HPD incubation in RBL-01 cells up until 4 hours, after which it showed a steady increase. Viability decreased in the AY27 cells with increased duration of HPD incubation. An increase in serum concentration in the medium resulted in an increase in viability for both cell lines. Both cell lines demonstrated fast initial uptake of HPD followed by slower uptake over the time studied. By 24 and 48 hours the AY27 cells contained twice the amount of methanol-extractable porphyrins as the RBL-01 cells. The initial morphologic change following HPD PDT was damage to mitochondria. Mitochondrial damage occurred immediately after PDT in the AY27 cells and 30 minutes after PDT in the RBL-01 cells. Both cell lines exhibited a similar progression of cell injury; however, morphologic damage was observed earlier after PDT and appeared more extensive in the AY27 cells.
Full text
PDF

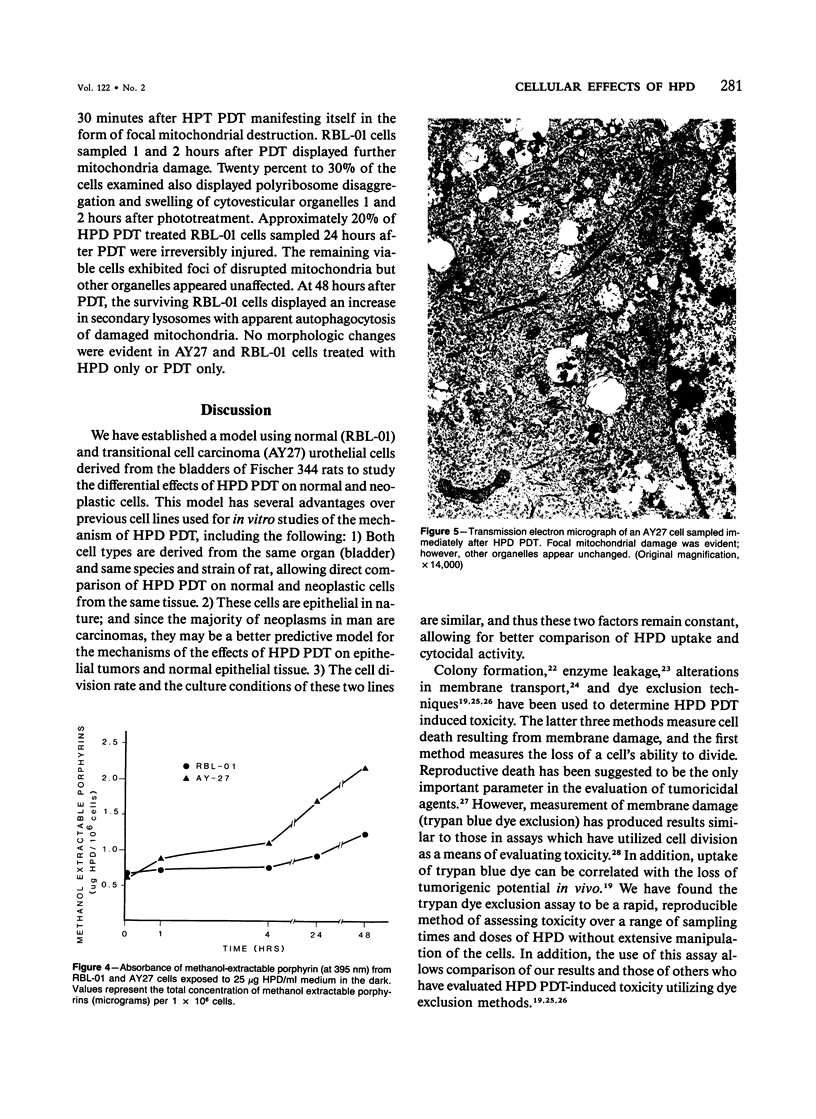
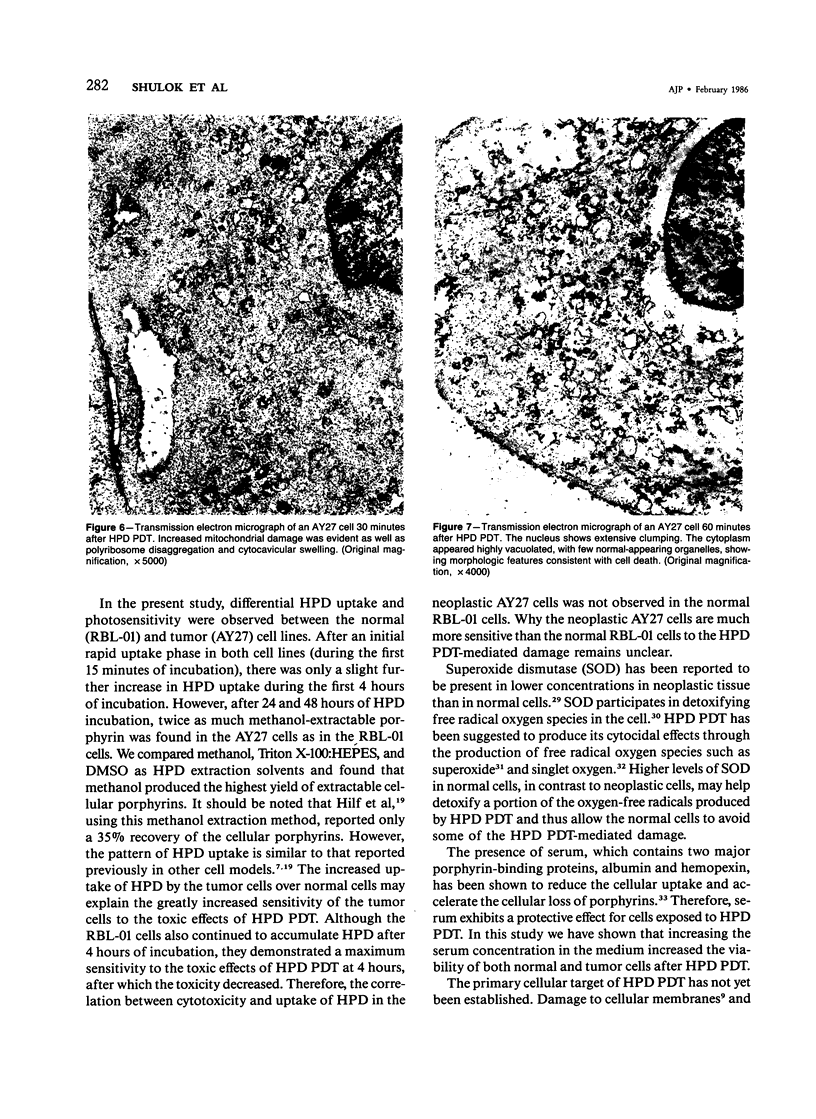

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoni A., Cubeddu R., De Silvestri S., Laporta P., Ambesi-Impiombato F. S., Esposito M., Mastrocinque M., Tramontano D. Effects of laser irradiation on hematoporphyrin-treated normal and transformed thyroid cells in culture. Cancer Res. 1983 May;43(5):2076–2080. [PubMed] [Google Scholar]
- Babcock M. S., Marino M. R., Gunning W. T., 3rd, Stoner G. D. Clonal growth and serial propagation of rat esophageal epithelial cells. In Vitro. 1983 May;19(5):403–415. doi: 10.1007/BF02619557. [DOI] [PubMed] [Google Scholar]
- Berns M. W., Dahlman A., Johnson F. M., Burns R., Sperling D., Guiltinan M., Siemens A., Walter R., Wright W., Hammer-Wilson M. In vitro cellular effects of hematoporphyrin derivative. Cancer Res. 1982 Jun;42(6):2325–2329. [PubMed] [Google Scholar]
- Berns M. W., Wilson M., Rentzepis P., Burns R., Wile A. Cell biology of hematoporphyrin derivative (HPD). Lasers Surg Med. 1983;2(3):261–266. doi: 10.1002/lsm.1900020309. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Superoxide formation by protoporphyrin as seen by spin trapping. FEBS Lett. 1979 Feb 1;98(1):18–20. doi: 10.1016/0014-5793(79)80141-6. [DOI] [PubMed] [Google Scholar]
- Christensen T., Sandquist T., Feren K., Waksvik H., Moan J. Retention and photodynamic effects of haematoporphyrin derivative in cells after prolonged cultivation in the presence of porphyrin. Br J Cancer. 1983 Jul;48(1):35–43. doi: 10.1038/bjc.1983.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Volden G., Moan J., Sandquist T. Release of lysosomal enzymes and lactate dehydrogenase due to hematoporphyrin derivative and light irradiation of NHIK 3025 cells in vitro. Ann Clin Res. 1982 Feb;14(1):46–52. [PubMed] [Google Scholar]
- Cohen S. M., Yang J. P., Jacobs J. B., Arai M., Fukushima S., Friedell G. H. Transplantation and cell culture of rat urinary bladder carcinoma. Invest Urol. 1981 Nov;19(3):136–141. [PubMed] [Google Scholar]
- Coppola A., Viggiani E., Salzarulo L., Rasile G. Ultrastructural changes in lymphoma cells treated with hematoporphyrin and light. Am J Pathol. 1980 Apr;99(1):175–192. [PMC free article] [PubMed] [Google Scholar]
- Diamond I., Granelli S. G., McDonagh A. F., Nielsen S., Wilson C. B., Jaenicke R. Photodynamic therapy of malignant tumours. Lancet. 1972 Dec 2;2(7788):1175–1177. doi: 10.1016/s0140-6736(72)92596-2. [DOI] [PubMed] [Google Scholar]
- Docchio F., Ramponi R., Sacchi C. A., Bottiroli G., Freitas I. Time-resolved fluorescence microscopy of hematoporphyrin-derivative in cells. Lasers Surg Med. 1982;2(1):21–28. doi: 10.1002/lsm.1900020103. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Datta-Gupta N., Mark E. H., Howard J. C. Induction of DNA damage by porphyrin photosensitizers. Cancer Res. 1981 Sep;41(9 Pt 1):3543–3545. [PubMed] [Google Scholar]
- Gibson S. L., Hilf R. Photosensitization of mitochondrial cytochrome c oxidase by hematoporphyrin derivative and related porphyrins in vitro and in vivo. Cancer Res. 1983 Sep;43(9):4191–4197. [PubMed] [Google Scholar]
- Gutter B., Speck W. T., Rosenkranz H. S. The photodynamic modification of DNA by hematoporphyrin. Biochim Biophys Acta. 1977 Mar 18;475(2):307–314. doi: 10.1016/0005-2787(77)90021-1. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Bellnier D. A., Ziring B., Dougherty T. J. Aspects of the cellular uptake and retention of hematoporphyrin derivative and their correlation with the biological response to PRT in vitro. Adv Exp Med Biol. 1983;160:129–138. doi: 10.1007/978-1-4684-4406-3_13. [DOI] [PubMed] [Google Scholar]
- Hilf R., Leakey P. B., Sollott S. J., Gibson S. L. Photodynamic inactivation of R3230AC mammary carcinoma in vitro with hematoporphyrin derivative: effects of dose, time, and serum on uptake and phototoxicity. Photochem Photobiol. 1983 Jun;37(6):633–642. doi: 10.1111/j.1751-1097.1983.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Kessel D. Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy. Photochem Photobiol. 1984 Jun;39(6):851–859. doi: 10.1111/j.1751-1097.1984.tb08871.x. [DOI] [PubMed] [Google Scholar]
- Klaunig J. E., Goldblatt P. J., Hinton D. E., Lipsky M. M., Knipe S. M., Trump B. F. Morphologic and functional studies of mouse hepatocytes in primary culture. Anat Rec. 1982 Nov;204(3):231–243. doi: 10.1002/ar.1092040308. [DOI] [PubMed] [Google Scholar]
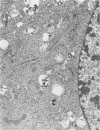
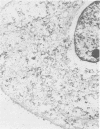
- Klaunig J. E., Selman S. H., Shulok J. R., Schafer P. J., Britton S. L., Goldblatt P. J. Morphologic studies of bladder tumors treated with hematoporphyrin derivative photochemotherapy. Am J Pathol. 1985 May;119(2):236–243. [PMC free article] [PubMed] [Google Scholar]
- Kohn K., Kessel D. On the mode of cytotoxic action of photo-activated porphyrins. Biochem Pharmacol. 1979 Aug 15;28(16):2465–2470. doi: 10.1016/0006-2952(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Moan J., Christensen T., Sommer S. The main photosensitizing components of hematoporphyrin derivative. Cancer Lett. 1982 Feb;15(2):161–166. doi: 10.1016/0304-3835(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Moan J., Johannessen J. V., Christensen T., Espevik T., McGhie J. B. Porphyrin-sensitized photoinactivation of human cells in vitro. Am J Pathol. 1982 Nov;109(2):184–192. [PMC free article] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J., Steen H. B., Feren K., Christensen T. Uptake of hematoporphyrin derivative and sensitized photoinactivation of C3H cells with different oncogenic potential. Cancer Lett. 1981 Dec;14(3):291–296. doi: 10.1016/0304-3835(81)90157-9. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Gray M. J., Silberman L., Lipson R. L. Identification of neoplastic versus normal cells in human cervical cell culture. Obstet Gynecol. 1974 May;43(5):635–639. [PubMed] [Google Scholar]
- Roper P. R., Drewinko B. Comparison of in vitro methods to determine drug-induced cell lethality. Cancer Res. 1976 Jul;36(7 Pt 1):2182–2188. [PubMed] [Google Scholar]
- Salet C., Moreno G. Photodynamic effects of haematoporphyrin on respiration and calcium uptake in isolated mitochondria. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Feb;39(2):227–230. doi: 10.1080/09553008114550261. [DOI] [PubMed] [Google Scholar]
- Schrek R. Utility and efficiency of viable cell counts. Cancer Res. 1979 Oct;39(10):4288–4288. [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]