Abstract
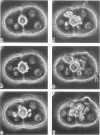
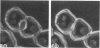
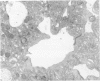
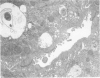
Actin filament based cell functions were examined in freshly isolated hepatocytes using phalloidin as an inhibitor. In particular, cell motility events, namely, surface bleb formation and canalicular contractile movements, were assessed and compared with morphologic changes in the cells. Phalloidin (in 0.1, 1.0, and 10.0 micrograms/ml dosages) was added to the culture medium 4 hours after isolation of the hepatocytes. Cell motility was recorded with time-lapse cinephotomicrography, and the morphologic changes were evaluated by phase-contrast optics and by transmission electron microscopy of serial sections. Membrane-bound pericanalicular cytoplasmic vacuoles appeared first, followed by cytoplasmic protrusions at the cell surface. Vacuolar membrane continuities with the canalicular membrane were noted, and later, with other regions of the cell surface, such that large tortuous irregular membrane-bound canals are seen on serial sectioning to link extracellular and canalicular spaces. These findings suggest a possible disturbance in membrane flow. Canalicular motility was greatly reduced and was dose-dependent. The time-based difference in the changes at the canalicular and sinusoidal surfaces may be indicative of different functions and/or sensitivities of the actin filaments within the liver cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubin M., Maurice M., Feldmann G., Erlinger S. Phalloidin-induced cholestasis in the rat: relation to changes in microfilaments. Gastroenterology. 1978 Sep;75(3):450–455. [PubMed] [Google Scholar]
- Elias E., Hruban Z., Wade J. B., Boyer J. L. Phalloidin-induced cholestasis: a microfilament-mediated change in junctional complex permeability. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2229–2233. doi: 10.1073/pnas.77.4.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S. W., Davies P. L. Ultrastructural localization of actin-like filaments in rat hepatocytes. Gastroenterology. 1975 Apr;68(4 Pt 1):765–774. [PubMed] [Google Scholar]
- Gabbiani G., Montesano R., Tuchweber B., Salas M., Orci L. Phalloidin-induced hyperplasia of actin filaments in rat hepatocytes. Lab Invest. 1975 Nov;33(5):562–569. [PubMed] [Google Scholar]
- Godman G. C., Miranda A. F., Deitch A. D., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. III. Zeiosis and movements at the cell surface. J Cell Biol. 1975 Mar;64(3):644–667. doi: 10.1083/jcb.64.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holborow E. J., Trenchev P. S., Dorling J., Webb J. Demostration of smooth muscle contractile protein antigens in liver and epithelial cells. Ann N Y Acad Sci. 1975 Jun 30;254:489–504. doi: 10.1111/j.1749-6632.1975.tb29196.x. [DOI] [PubMed] [Google Scholar]
- Imanari H., Kuroda H., Tamura K. Microfilaments around the bile canaliculi in patients with intrahepatic cholestasis. Gastroenterol Jpn. 1981;16(2):168–173. doi: 10.1007/BF02774391. [DOI] [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Mooney J. S., Ockner R. K., Adler R. D. Alterations in hepatic pericanalicular cytoplasm during enhanced bile secretory activity. Lab Invest. 1979 Apr;40(4):512–517. [PubMed] [Google Scholar]
- Kane A. B., Young E. E., Schanne F. A., Farber J. L. Calcium dependence of phalloidin-induced liver cell death. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1177–1180. doi: 10.1073/pnas.77.2.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishes B. A., Williams G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. 1. The effect of insulin. In Vitro. 1976 Jul;12(7):521–532. doi: 10.1007/BF02796495. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Namihisa T., Tamura K., Saifuku K., Imanari H., Kuroda H., Kanaoka Y., Okamoto Y., Sekine T. Fluorescent staining of microfilaments with heavy meromyosin labeled with N-(7-dimethylamino-4-methylcoumarinyl) maleimide. J Histochem Cytochem. 1980 Apr;28(4):335–338. doi: 10.1177/28.4.6989895. [DOI] [PubMed] [Google Scholar]
- Oda M., Price V. M., Fisher M. M., Phillips M. J. Ultrastructure of bile canaliculi, with special reference to the surface coat and the pericanalicular web. Lab Invest. 1974 Oct;31(4):314–323. [PubMed] [Google Scholar]
- Oshio C., Phillips M. J. Contractility of bile canaliculi: implications for liver function. Science. 1981 May 29;212(4498):1041–1042. doi: 10.1126/science.7015506. [DOI] [PubMed] [Google Scholar]
- Petzinger E. Competitive inhibition of the uptake of demethylphalloin by cholic acid in isolated hepatocytes. Evidence for a transport competition rather than a binding competition. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jul;316(4):345–349. doi: 10.1007/BF00501368. [DOI] [PubMed] [Google Scholar]
- Petzinger E., Frimmer M. Comparative studies on the uptake of 14C-bile acids and 3H-demethylphalloin in isolated rat liver cells. Arch Toxicol. 1980 Mar;44(1-3):127–135. doi: 10.1007/BF00303189. [DOI] [PubMed] [Google Scholar]
- Phillips M. J., Oda M., Mak E., Fisher M. M., Jeejeebhoy K. N. Microfilament dysfunction as a possible cause of intrahepatic cholestasis. Gastroenterology. 1975 Jul;69(1):48–58. [PubMed] [Google Scholar]
- Phillips M. J., Oshio C., Miyairi M., Smith C. R. Intrahepatic cholestasis as a canalicular motility disorder. Evidence using cytochalasin. Lab Invest. 1983 Feb;48(2):205–211. [PubMed] [Google Scholar]
- Russo M. A., Kane A. B., Farber J. L. Ultrastruct pathology of phalloidin-intoxicated hepatocytes in the presence and absence of extracellular calcium. Am J Pathol. 1982 Nov;109(2):133–144. [PMC free article] [PubMed] [Google Scholar]
- Sato T. A modified method for lead staining of thin sections. J Electron Microsc (Tokyo) 1968;17(2):158–159. [PubMed] [Google Scholar]
- Simons T. J. A method for estimating free Ca within human red blood cells, with an application to the study of their Ca-dependent K permeability. J Membr Biol. 1982;66(3):235–247. doi: 10.1007/BF01868498. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Miyairi M., Oshio C., Smith C. R., Phillips M. J. Phalloidin alters bile canalicular contractility in primary monolayer cultures of rat liver. Gastroenterology. 1983 Aug;85(2):245–253. [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5613–5617. doi: 10.1073/pnas.74.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Stockem W., Weber K. Cytoplasmic streaming in Amoeba proteus is inhibited by the actin-specific drug phalloidin. Exp Cell Res. 1978 Sep;115(2):451–454. doi: 10.1016/0014-4827(78)90307-5. [DOI] [PubMed] [Google Scholar]
- Weiss E., Sterz I., Frimmer M., Kroker R. Electron microscopy of isolated rat hepatocytes before and after treatment with phalloidin. Beitr Pathol. 1973 Dec;150(4):345–356. doi: 10.1016/s0005-8165(73)80085-x. [DOI] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]
- Wu P. C., Lai C. L., Lam K. C., Ho J. Prednisolone in HBsAg-positive chronic active hepatitis: histologic evaluation in a controlled prospective study. Hepatology. 1982 Nov-Dec;2(6):777–783. doi: 10.1002/hep.1840020605. [DOI] [PubMed] [Google Scholar]