Abstract
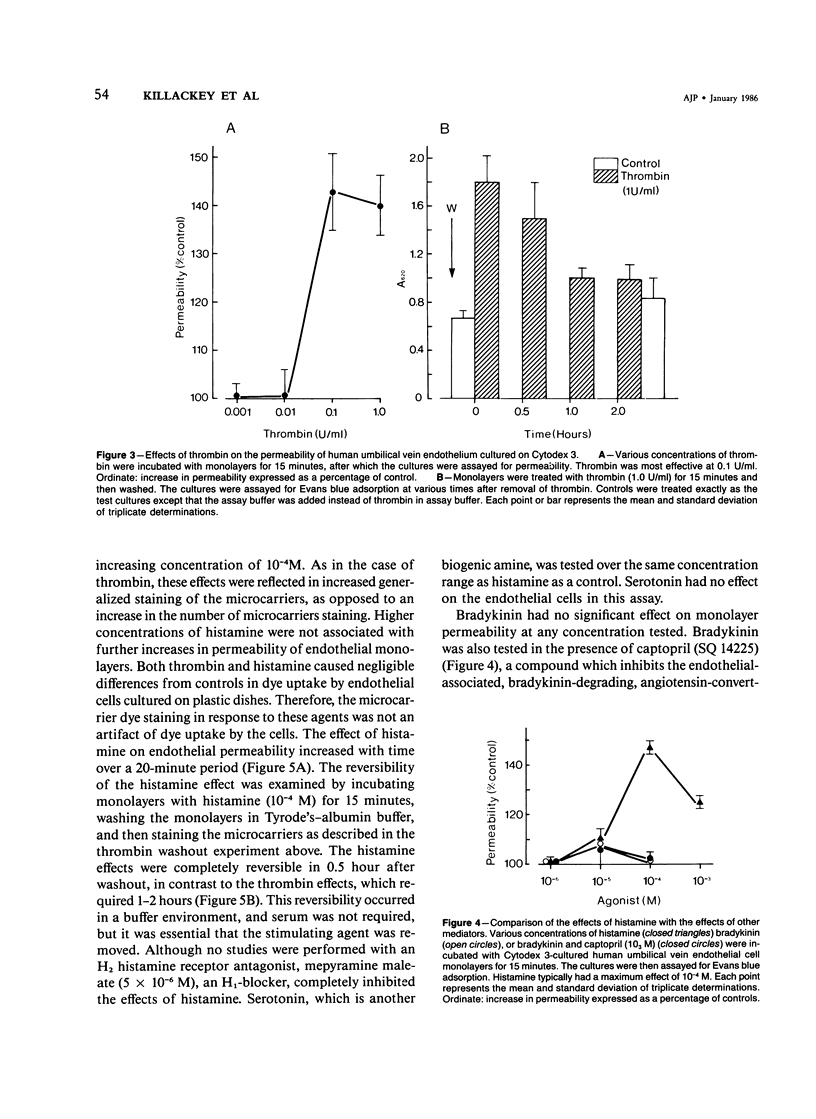
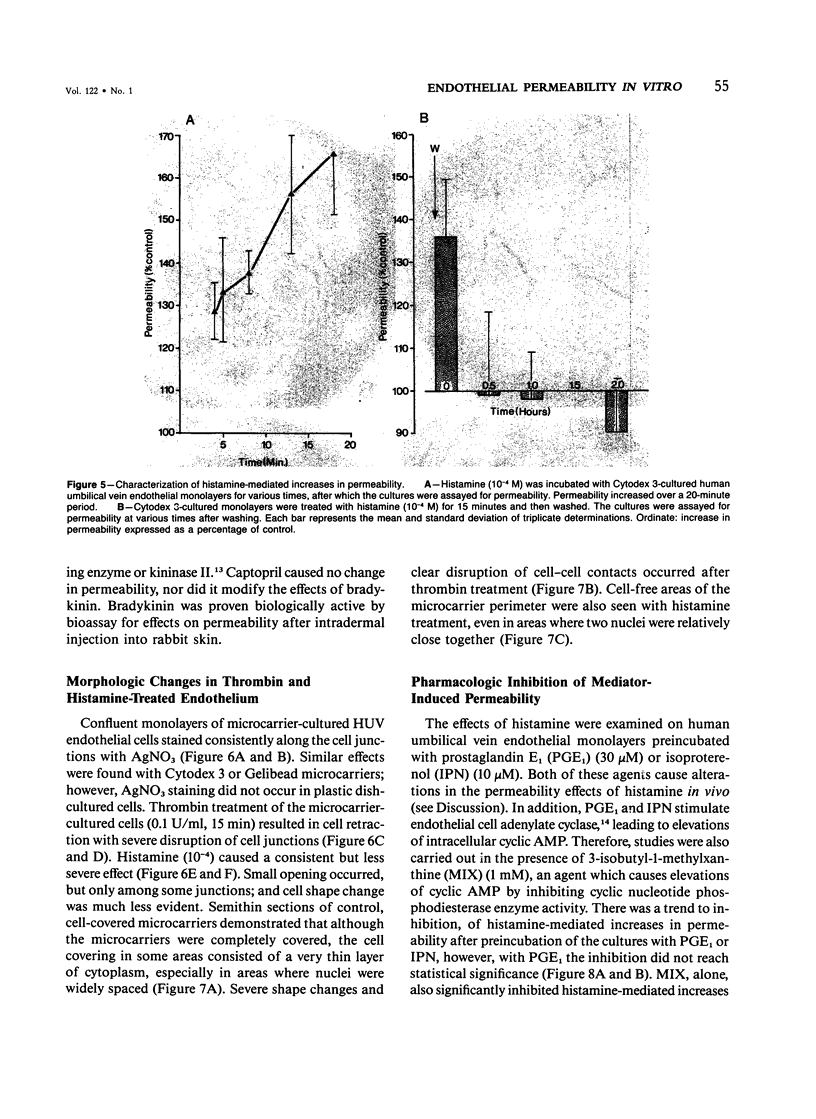
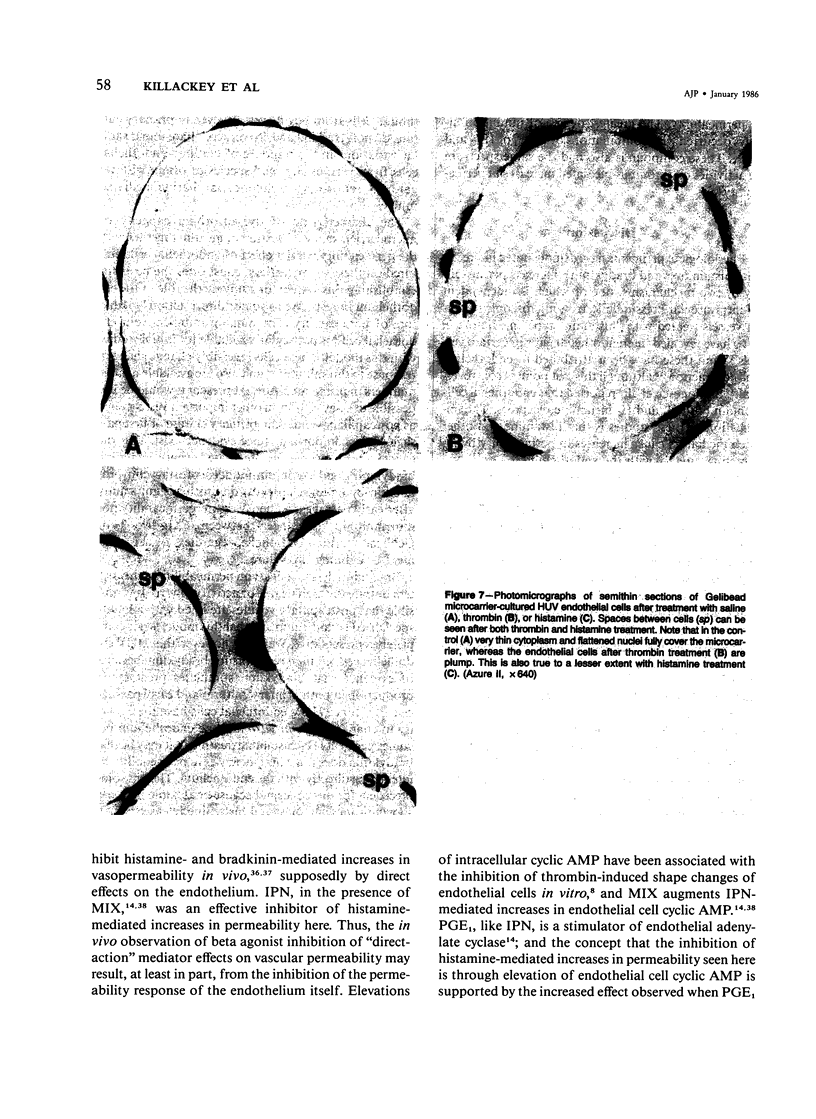
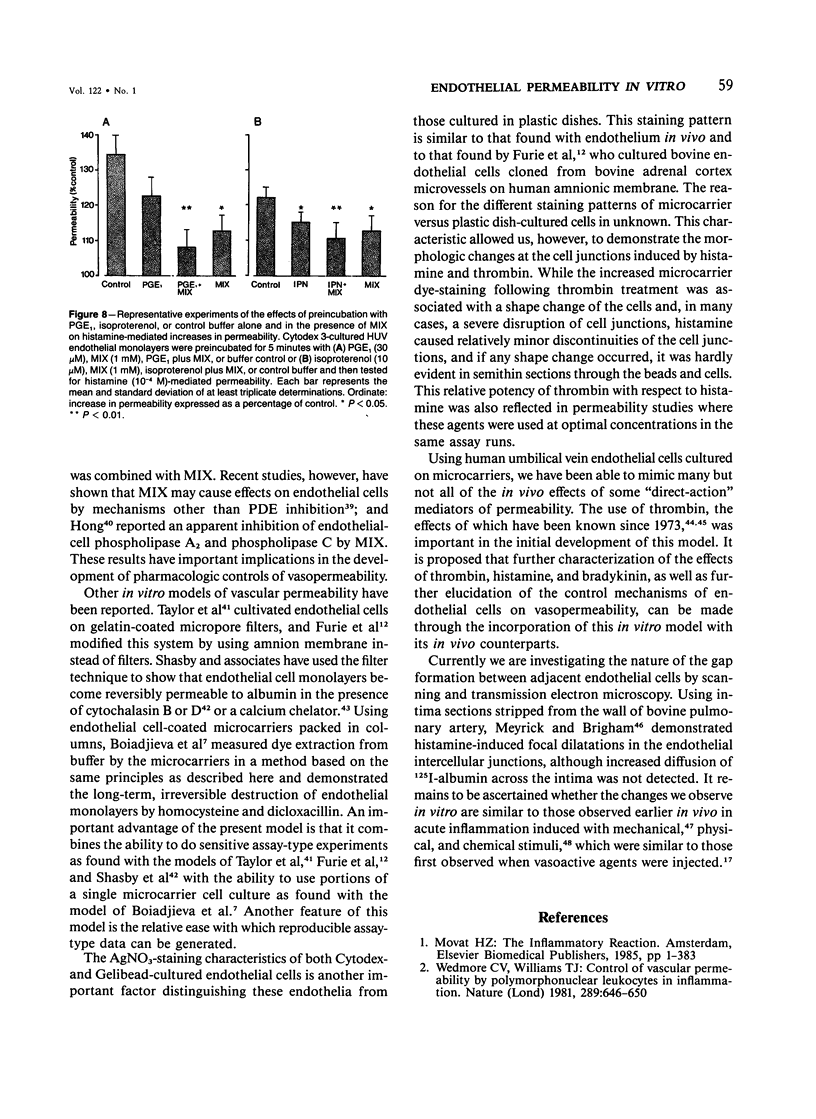
The permeability response of endothelial monolayers to some "direct-action" type mediators of vasopermeability were studied in vitro. Endothelial cells, cultured to confluence on denatured collagen-coated dextran microcarriers or gelatin microcarriers, prevented staining of the microcarriers with Evans blue dye. Increases in staining, as determined by the spectrophotometric quantitation of the dye after extraction from the microcarriers with formamide, occurred after treatment of human umbilical vein endothelium with histamine (10(-5) M) or thrombin (0.1 U/ml). These increases in monolayer permeability were reversible. Neither bradykinin nor serotonin had any effect in this system. Endothelial monolayers cultured this way consistently stained with silver nitrate at the cell junction areas. Monolayer response to histamine was characterized morphologically by small openings which occurred randomly along the cell junctions; while with thrombin, the spaces, which had developed at junctions, occurred to a greater extent. Prostaglandin E1 (30 microM) and isoproterenol (10 microM), in the presence of 3-isobutyl-1-methylxanthine (1 mM), partially inhibited histamine- and thrombin-mediated changes in permeability. This model responds to certain vasopermeability-altering agents in a manner similar to that of the microcirculation. These studies support the concept that the vasopermeability enhancing effect of histamine in vivo results, in part, from a direct effect on the endothelium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boiadjieva S., Hallberg C., Högström M., Busch C. Methods in laboratory investigation. Exclusion of trypan blue from microcarriers by endothelial cells: an in vitro barrier function test. Lab Invest. 1984 Feb;50(2):239–246. [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Macfarlane D. E., Hoak J. C. Prostacyclin biosynthesis in vascular endothelium is not inhibited by cyclic AMP. Studies with 3-isobutyl-1-methylxanthine and forskolin. Thromb Res. 1982 Dec 1;28(5):637–647. doi: 10.1016/0049-3848(82)90155-4. [DOI] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Evensen S. A. Injury to cultured endothelial cells: the role of lipoproteins and thrombo-active agents. Haemostasis. 1979;8(3-5):203–210. doi: 10.1159/000214312. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdal K. S., Evensen S. A., Nilsen E. Thrombin-induced shape changes of cultured endothelial cells: metabolic and functional observations. Thromb Res. 1983 Oct 1;32(1):57–66. doi: 10.1016/0049-3848(83)90154-8. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Grega G. J., Kline R. L., Dobbins D. E., Haddy F. J. Mechanisms of edema formation by histamine administered locally into canine forelimbs. Am J Physiol. 1972 Nov;223(5):1165–1171. doi: 10.1152/ajplegacy.1972.223.5.1165. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Hong S. L. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb Res. 1980 Jun 15;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- Hong S. L. Inhibition of prostacyclin synthesis in endothelial cells by methylisobutylxanthine is not mediated through elevated cAMP level. Biochim Biophys Acta. 1983 Dec 20;754(3):258–263. doi: 10.1016/0005-2760(83)90140-6. [DOI] [PubMed] [Google Scholar]
- Hopkins N. K., Gorman R. R. Regulation of endothelial cell cyclic nucleotide metabolism by prostacyclin. J Clin Invest. 1981 Feb;67(2):540–546. doi: 10.1172/JCI110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a. Crit Rev Immunol. 1981 Feb;1(4):321–366. [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Hanahan D. J., Pinckard R. N. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1984 Jan;50(1):16–25. [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Satouchi K., Hanahan D. J., Pinckard R. N. Vasoactive properties of acetyl glyceryl ether phosphorylcholine and analogues. Lab Invest. 1982 Apr;46(4):422–427. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973 Apr;142(4):1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Johnston M. G., Walker M. A. Lymphatic endothelial and smooth-muscle cells in tissue culture. In Vitro. 1984 Jul;20(7):566–572. doi: 10.1007/BF02639772. [DOI] [PubMed] [Google Scholar]
- Kline R. L., Scott J. B., Haddy F. J., Grega G. J. Mechanism of edema formation in canine forelimbs by locally administered bradykinin. Am J Physiol. 1973 Nov;225(5):1051–1056. doi: 10.1152/ajplegacy.1973.225.5.1051. [DOI] [PubMed] [Google Scholar]
- Laposata M., Dovnarsky D. K., Shin H. S. Thrombin-induced gap formation in confluent endothelial cell monolayers in vitro. Blood. 1983 Sep;62(3):549–556. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. ACUTE INFLAMMATION. THE EARLIEST FINE STRUCTURAL CHANGES AT THE BLOOD-TISSUE BARRIER. Lab Invest. 1963 Sep;12:895–910. [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano Y., Sawasaki Y., Takahashi K., Goto T. Cultivation of arterial endothelial cells from human umbilical cord. Experientia. 1983 Oct 15;39(10):1144–1146. doi: 10.1007/BF01943152. [DOI] [PubMed] [Google Scholar]
- Marciniak D. L., Dobbins D. E., Maciejko J. J., Scott J. B., Haddy F. J., Grega G. J. Antagonism of histamine edema formation by catecholamines. Am J Physiol. 1978 Feb;234(2):H180–H185. doi: 10.1152/ajpheart.1978.234.2.H180. [DOI] [PubMed] [Google Scholar]
- McDonald R. I., Shepro D., Rosenthal M., Booyse F. M. Properties of cultured endothelial cells. Ser Haematol. 1973;6(4):469–478. [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Increased permeability associated with dilatation of endothelial cell junctions caused by histamine in intimal explants from bovine pulmonary artery. Exp Lung Res. 1984;6(1):11–25. doi: 10.3109/01902148409087892. [DOI] [PubMed] [Google Scholar]
- Morley J., Page C. P., Paul W. Inflammatory actions of platelet activating factor (Pafacether) in guinea-pig skin. Br J Pharmacol. 1983 Nov;80(3):503–509. doi: 10.1111/j.1476-5381.1983.tb10722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Hutchings A. Prostacyclin release stimulated by thrombin or bradykinin in porcine endothelial cells cultured from aorta and umbilical vein. Thromb Res. 1983 Jan 15;29(2):115–124. doi: 10.1016/0049-3848(83)90133-0. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Grega G. J., Svensjö E. The role of beta-receptor agonists in the inhibition of pulmonary edema. Ann N Y Acad Sci. 1982;384:544–557. doi: 10.1111/j.1749-6632.1982.tb21399.x. [DOI] [PubMed] [Google Scholar]
- Prasad C. M., Adamski S. W., Svensjö E., Grega G. J. Pharmacological modification of the edema produced by combined infusions of prostaglandin E1 and bradykinin in canine forelimbs. J Pharmacol Exp Ther. 1982 Feb;220(2):293–298. [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafelson M. E., Jr, Hoveke T. P., Booyse F. M. The molecular biology of platelet-platelet and platelet-endothelial interactions. Ser Haematol. 1973;6(3):367–381. [PubMed] [Google Scholar]
- Rubin B., Laffan R. J., Kotler D. G., O'Keefe E. H., Demaio D. A., Goldberg M. E. SQ 14,225 (D-3-mercapto-2-methylpropanoyl-l-proline), a novel orally active inhibitor of angiotensin I-converting enzyme. J Pharmacol Exp Ther. 1978 Feb;204(2):271–280. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Heltianu C., Antohe F., Simionescu M. Endothelial cell receptors for histamine. Ann N Y Acad Sci. 1982;401:132–149. doi: 10.1111/j.1749-6632.1982.tb25713.x. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Price T. H., Schwartz S. M., Dale D. C. Neutrophil-endothelial cell interactions on endothelial monolayers grown on micropore filters. J Clin Invest. 1981 Feb;67(2):584–587. doi: 10.1172/JCI110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]