Abstract
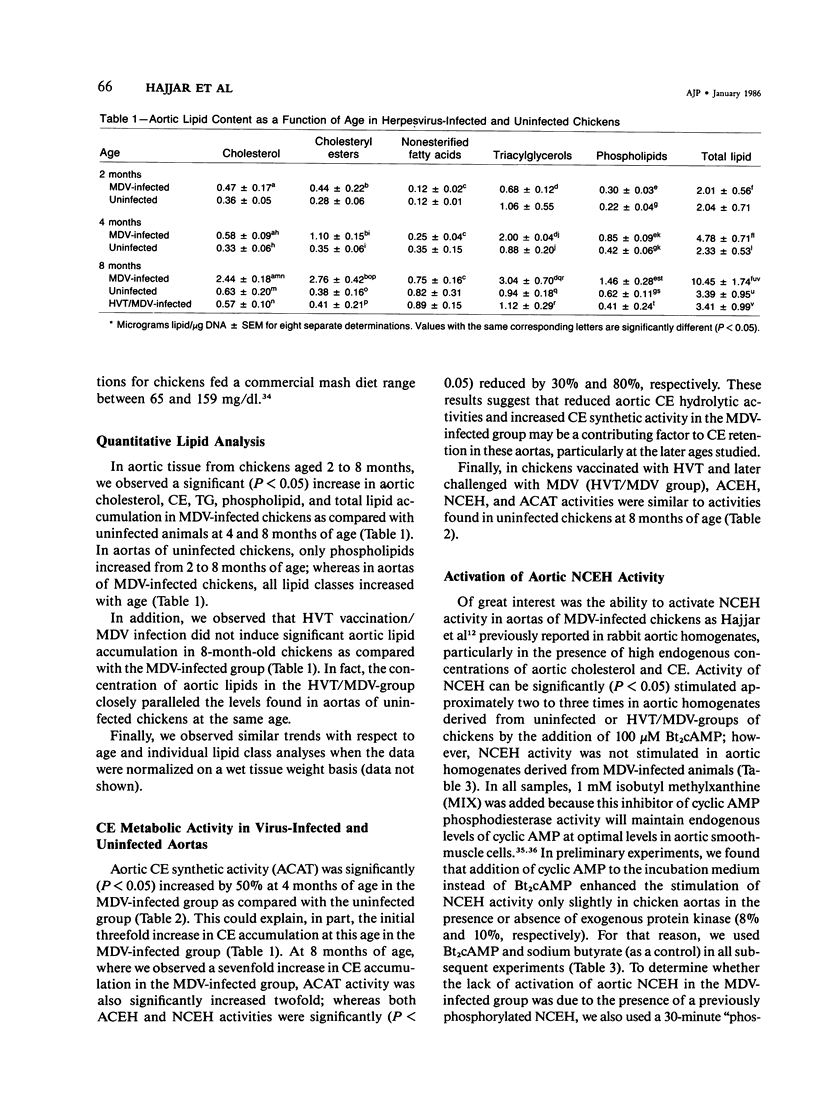
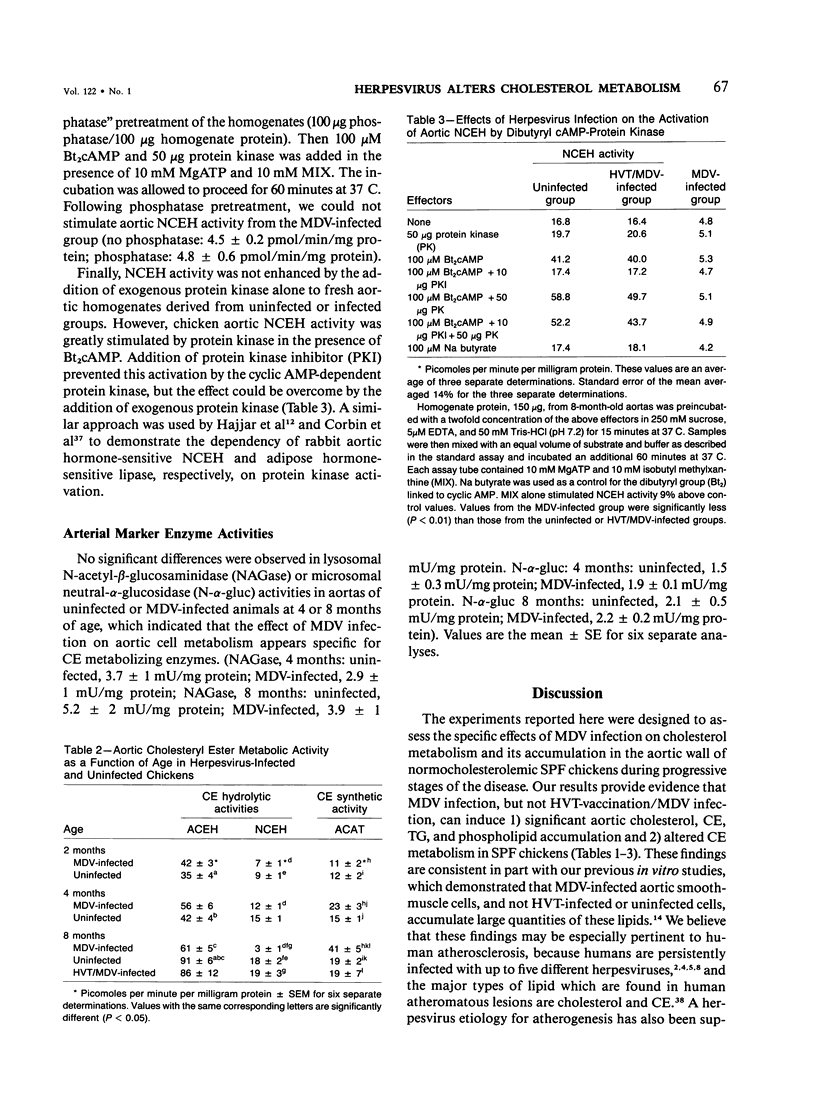
Infection of normocholesterolemic, specific-pathogen-free chickens with Marek's disease herpesvirus (MDV) has been shown histologically to lead to chronic atherosclerosis like that in humans. The development of herpesvirus-induced atherosclerosis in vivo and the presence of specific Marek's antigen within aortic cells suggested that MDV infection may modify lipid metabolism and lead to significant lipid accumulation. Experiments reported herein were designed to determine the types and quantity of lipid present in aortas from MDV-infected and uninfected chickens between 2 and 8 months of age following infection and assess one possible mechanism of lipid accumulation by evaluating the effect of MDV infection on aortic cholesterol and cholesteryl ester (CE) metabolism. Chromatographic-fluorometric analyses indicated that at 4 and 8 months of age after MDV inoculation, MDV-infected animals had a significant (P less than 0.05) two-fold to threefold increase in total aortic lipid accumulation characterized by significant increases in cholesterol, CE, triacylglycerol, and phospholipid as compared with aortas from uninfected animals. At 8 months of age, similar increases in aortic lipid accumulation were observed in MDV-infected animals as compared with those animals vaccinated with turkey herpesvirus and later challenged with MDV. CE synthetic activity was increased significantly by 50% at 4 months of age in the MDV-infected group as compared with the uninfected group, which could explain the initial increase in CE accumulation. By 8 months of age, the authors also observed a twofold increase in CE synthetic activity and a 30% and 80% reduction in lysosomal and cytoplasmic CE hydrolytic activities, respectively, in aortas of MDV-infected chickens as compared to controls. Moreover, infection with MDV blocked the activation of cytoplasmic CE hydrolytic activity by dibutyryl cyclic AMP or exogenous cyclic AMP-dependent protein kinase. Taken together, these results suggest that lipid accretion in aortas of MDV-infected chickens results, in part, from alterations in cholesterol/CE metabolism during early stages of the disease. These findings support the hypothesis that human atherosclerosis may result from specific herpesvirus infection which can alter lipid metabolism and lead to lipid accretion.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Dales S. Biogenesis of poxviruses: glycolipid metabolism in vaccinia-infected cells. Virology. 1978 Jan;84(1):108–117. doi: 10.1016/0042-6822(78)90222-2. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Phospholipid metabolism of herpesvirus-infected and uninfected rabbit kidney cells. Virology. 1971 Jul;45(1):252–264. doi: 10.1016/0042-6822(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondjers G., Björkerud S. Fluorometric determination of cholesterol and cholesteryl ester in tissue on the nanogram level. Anal Biochem. 1971 Aug;42(2):363–371. doi: 10.1016/0003-2697(71)90049-2. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979 Sep;82(3):597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W., Adldinger H. K., Kahn D. E. Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek's disease. Avian Dis. 1970 May;14(2):219–233. [PubMed] [Google Scholar]
- Calnek B. W. Effects of passive antibody on early pathogenesis of Marek's disease. Infect Immun. 1972 Aug;6(2):193–198. doi: 10.1128/iai.6.2.193-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carić-Lazar M., Schwarz R. T., Scholtissek C. Influence of the infection with lipid-containing viruses on the metabolism and pools of phospholipid precursors in animal cells. Eur J Biochem. 1978 Nov 15;91(2):351–361. doi: 10.1111/j.1432-1033.1978.tb12687.x. [DOI] [PubMed] [Google Scholar]
- Castelli W. P. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984 Feb 27;76(2A):4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Cole R. K. Studies on genetic resistance to Marek's disease. Avian Dis. 1968 Feb;12(1):9–28. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M., Walsh D. A., Krebs E. G. Activation of adipose tissue lipase by skeletal muscle cyclic adenosine 3',5'- monophosphate-stimulated protein kinase. J Biol Chem. 1970 Sep 25;245(18):4849–4851. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Minick C. R., Litrenta M. M. Herpesvirus-induced atherosclerosis in chickens. Fed Proc. 1983 May 15;42(8):2476–2479. [PubMed] [Google Scholar]
- Fabricant C. G., Hajjar D. P., Minick C. R., Fabricant J. Herpesvirus infection enhances cholesterol and cholesteryl ester accumulation in cultured arterial smooth muscle cells. Am J Pathol. 1981 Nov;105(2):176–184. [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., Mated N., Shio H., Minick C. R., Fowler S. D. Lipoprotein-heparin-fibronectin-denatured collagen complexes enhance cholesteryl ester accumulation in macrophages. J Cell Biol. 1984 Oct;99(4 Pt 1):1266–1274. doi: 10.1083/jcb.99.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. P., Nazerian K., Velicer L. F., Kung H. J. Extensive homology exists between Marek disease herpesvirus and its vaccine virus, herpesvirus of turkeys. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3365–3369. doi: 10.1073/pnas.81.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Garcia-Palmieri M. R., Kagan A., Kannel W. B., Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974 Sep;27(7-8):329–344. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fowler S., Minick C. R. Endothelium modifies the altered metabolism of the injured aortic wall. Am J Pathol. 1981 Jan;102(1):28–39. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Minick C. R., Fowler S. Arterial neutral cholesteryl esterase. A hormone-sensitive enzyme distinct from lysosomal cholesteryl esterase. J Biol Chem. 1983 Jan 10;258(1):192–198. [PubMed] [Google Scholar]
- Hajjar D. P. Prostacyclin and cyclic nucleotides interact to modulate arterial cholesteryl ester metabolism. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:605–614. [PubMed] [Google Scholar]
- Hajjar D. P., Weksler B. B., Falcone D. J., Hefton J. M., Tack-Goldman K., Minick C. R. Prostacyclin modulates cholesteryl ester hydrolytic activity by its effect on cyclic adenosine monophosphate in rabbit aortic smooth muscle cells. J Clin Invest. 1982 Sep;70(3):479–488. doi: 10.1172/JCI110639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Weksler B. B. Metabolic activity of cholesteryl esters in aortic smooth muscle cells is altered by prostaglandins I2 and E2. J Lipid Res. 1983 Sep;24(9):1176–1185. [PubMed] [Google Scholar]
- Hajjar D. P., Wight T. N., Smith S. C. Lipid accumulation and ultrastructural change within the aortic wall during early spontaneous atherogenesis. Am J Pathol. 1980 Sep;100(3):683–705. [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Fowler S., de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res. 1980 Nov;21(8):961–969. [PubMed] [Google Scholar]
- Haley N. J., Shio H., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. I. Resolution of aortic cell populations by metrizamide density gradient centrifugation. Lab Invest. 1977 Sep;37(3):287–296. [PubMed] [Google Scholar]
- Hojnacki J. L., Smith S. C. Separation of six lipid classes on one thin-layer chromatogram. J Chromatogr. 1974 Apr 10;90(2):364–367. doi: 10.1016/s0021-9673(00)92542-1. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Khoo J. C., Steinberg D., Huang J. J., Vagelos P. R. Triglyceride, diglyceride, monoglyceride, and cholesterol ester hydrolases in chicken adipose tissue activated by adenosine 3':5'-Monophosphate-dependent protein kinase. Chromatographic resolution and immunochemical differentiation from lipoprotein lipase. J Biol Chem. 1976 May 25;251(10):2882–2890. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mark-Malchoff D., Marinetti G. V., Hare G. D., Meisler A. Characterization of phosphatidylthreonine in polyoma virus transformed fibroblasts. Biochemistry. 1978 Jun 27;17(13):2684–2688. doi: 10.1021/bi00606a035. [DOI] [PubMed] [Google Scholar]
- Mark-Malchoff D., Marinetti G. V., Hare J. D., Meisler A. Cholesterol content and metabolism in normal and polyoma virus-transformed hamster embryo fibroblasts. Exp Cell Res. 1979 Feb;118(2):377–381. doi: 10.1016/0014-4827(79)90161-7. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Petrie B. L., Dreesman G. R., Burek J., McCollum C. H., DeBakey M. E. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983 Sep 17;2(8351):644–647. doi: 10.1016/s0140-6736(83)92529-1. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. Spontaneous and experimentally induced arterial lesions. I. An ultrastructural survey of the normal chicken aorta. Lab Invest. 1970 Feb;22(2):166–183. [PubMed] [Google Scholar]
- Nicolosi R. J., Smith S. C., Santerre R. F. Simultaneous fluorometric analysis of five lipid classes on thin-layer chromatograms. J Chromatogr. 1971 Aug 5;60(1):111–117. [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Papahadjopoulos D. Cholesterol and cell membrane function: a hypothesis concerning etiology of atherosclerosis. J Theor Biol. 1974 Feb;43(2):329–337. doi: 10.1016/s0022-5193(74)80064-0. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Khoo J. C., Steinberg D. Cholesterol esterase in rat adipose tissue and its activation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jun 25;250(12):4505–4511. [PubMed] [Google Scholar]
- Pittman R. C., Steinberg D. Activatable cholesterol esterase and triacylglycerol lipase activities of rat adrenal and their relationship. Biochim Biophys Acta. 1977 Jun 22;487(3):431–444. doi: 10.1016/0005-2760(77)90214-4. [DOI] [PubMed] [Google Scholar]
- Schroder E. W., Merrick J. M. Alterations in glycosphingolipid patterns in a line of African green monkey kidney cells infected with herpesvirus. J Virol. 1979 Dec;32(3):734–740. doi: 10.1128/jvi.32.3.734-740.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson D. L., Fletcher T., Groves G., Hurley B., Sloan S. Hydrolysis of triolein, cholesterol oleate, and 4-methylumbelliferyl stearate by acid and neutral ester hydrolases (lipases) from pigeon adipose tissue: effect of cAMP-dependent protein kinase. Can J Biochem. 1981 Jun;59(6):418–429. doi: 10.1139/o81-058. [DOI] [PubMed] [Google Scholar]
- Shio H., Haley N. J., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. II. Morphometric analysis of lipid-filled lysosomes and lipid droplets in aortic cell populations. Lab Invest. 1978 Oct;39(4):390–397. [PubMed] [Google Scholar]
- Shio H., Haley N. J., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl ester. Lab Invest. 1979 Aug;41(2):160–167. [PubMed] [Google Scholar]
- VAHOUNY G. V., WEERSING S., TREADWELL C. R. MICELLAR-SOLUBILIZED SUBSTRATES AND CHOLESTEROL ESTERASE ACTIVITY IN VITRO. Arch Biochem Biophys. 1964 Jul;107:7–15. doi: 10.1016/0003-9861(64)90262-0. [DOI] [PubMed] [Google Scholar]


