Abstract
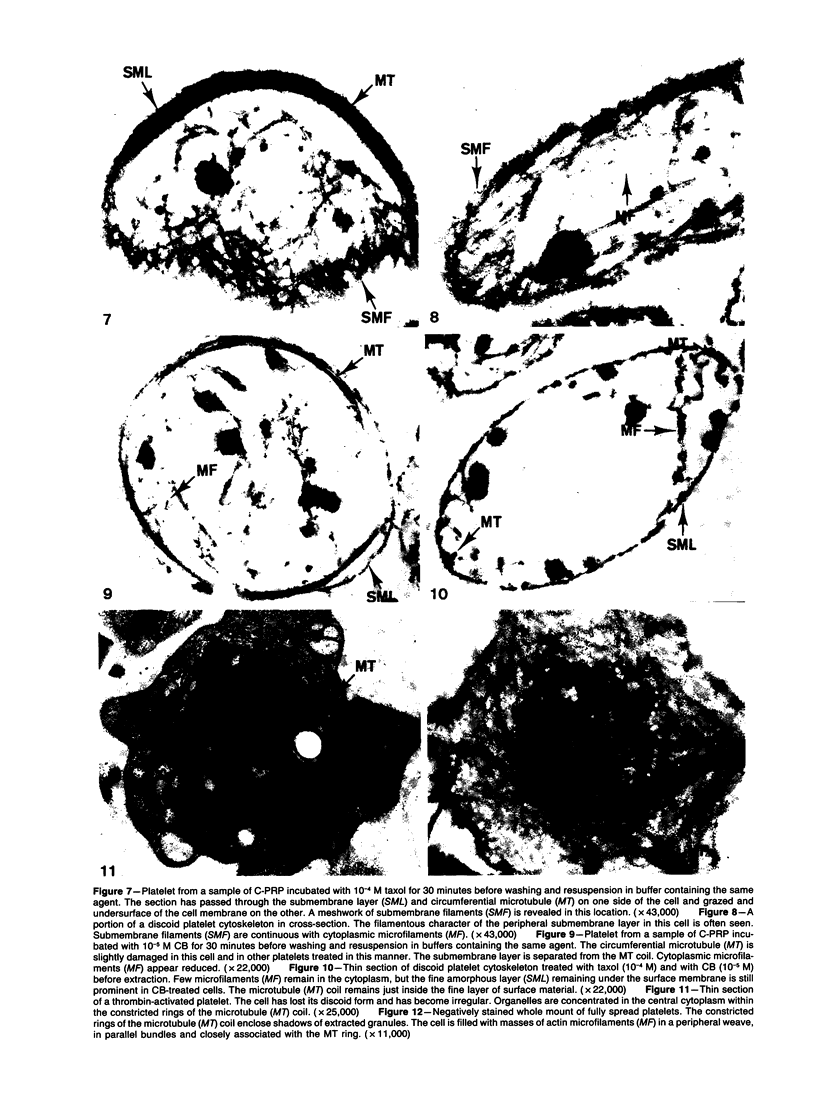
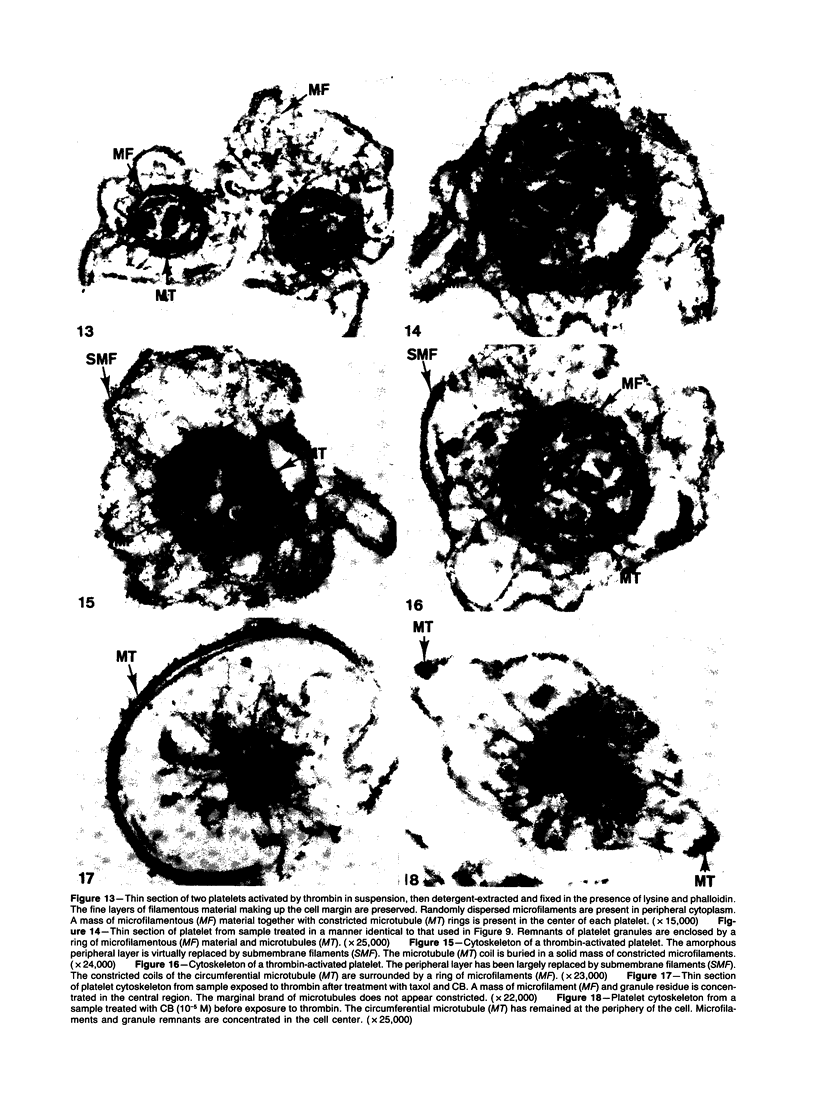
The present study has employed lysine, phalloidin, and a low concentration of osmic acid to protect the actin cytoskeleton of resting and thrombin-activated platelets during detergent extraction and fixation in suspension. Thin sections of resting platelets revealed a fine amorphous layer containing a few short actin filaments mimicking discoid shape and a randomly dispersed network of actin polymers in the cytoplasm. Following thrombin activation, the cytoskeleton consisted of a peripheral layer of submembrane actin filaments following the contour of shape change, a variable number of filaments in peripheral cytoplasm and extending into pseudopods, and a concentric mass of actin filaments with constricted microtubule rings in cell centers. Prior treatment with cytochalasin B (CB) appeared to reduce the number of actin filaments in resting platelets. Thrombin activation of CB-treated cells resulted in separation of actin filaments, which became concentrated in cell centers, from microtubule coils remaining at the cell periphery. The appearance of detergent-extracted cytoskeletons of platelet actin protected during fixation in suspension by lysine and phalloidin was remarkably similar to that of resting or CB-treated platelets before and after thrombin activation when viewed in conventionally prepared thin sections without exposure to detergent during fixation. The advantage of the new procedure is revelation of the actin filament organization in suspended platelets, which is obscured by the usual glutaraldehyde-osmic acid fixation technique.
Full text
PDF


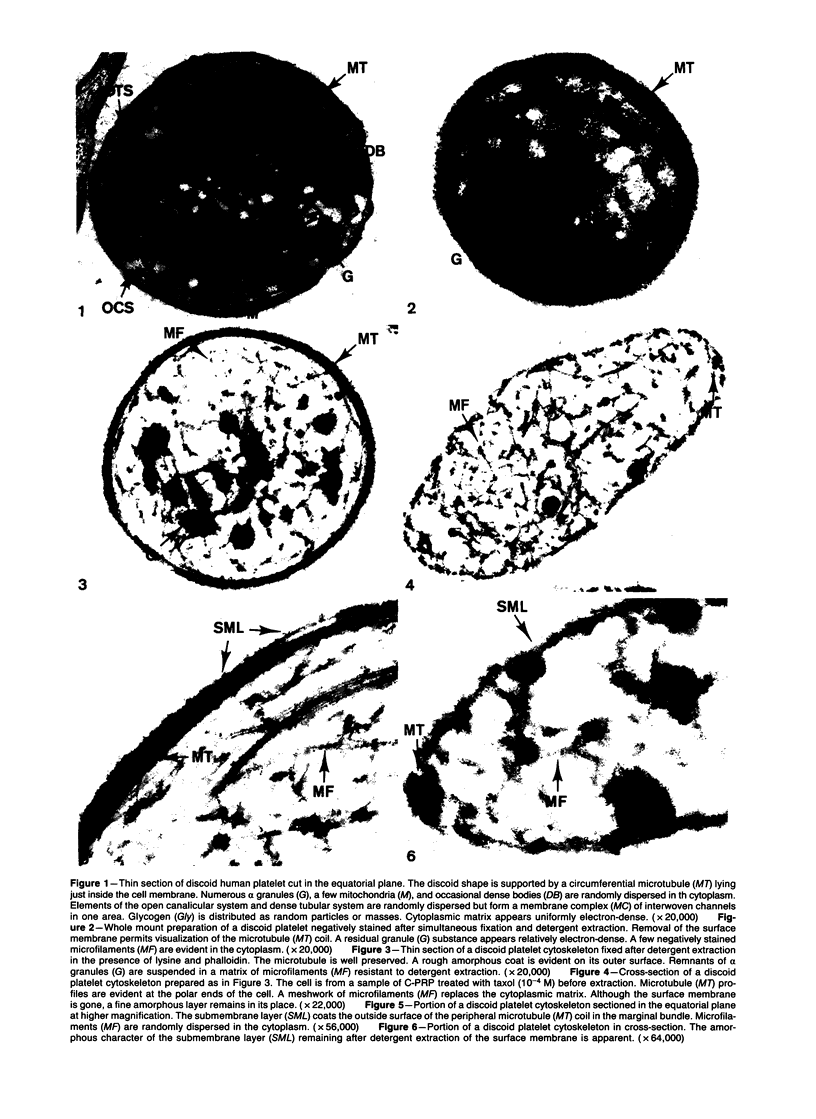



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Zacharski L. R., Widirstky S. T., Rosenstein R., Zaitlin L. M., Burgess D. R. Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. J Cell Biol. 1979 Oct;83(1):126–142. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M., Tavassoli M. OTO method for preservation of actin filaments in electron microscopy. J Histochem Cytochem. 1981 May;29(5):682–683. doi: 10.1177/29.5.6894766. [DOI] [PubMed] [Google Scholar]
- Casella J. F., Flanagan M. D., Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981 Sep 24;293(5830):302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Gerrard J. M., White J. G. Ultrastructure of clots during isometric contraction. J Cell Biol. 1982 Jun;93(3):775–787. doi: 10.1083/jcb.93.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio L. M., Tilney L. G. Phalloidin enhances actin assembly by preventing monomer dissociation. J Cell Biol. 1984 Aug;99(2):529–535. doi: 10.1083/jcb.99.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Boyles J. K., Reynolds C. C., Phillips D. R. Actin filament content and organization in unstimulated platelets. J Cell Biol. 1984 Jun;98(6):1985–1991. doi: 10.1083/jcb.98.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981 Aug 13;292(5824):650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Polymerization and organization of actin filaments within platelets. Semin Hematol. 1983 Oct;20(4):243–260. [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicquaud C., Loranger A. La phalloïdine protège la F-actine contre les effets destructeurs de l'acide osmique. II. Protection par la phalloïdine de la F-actine traitée aux aldéhydes. Eur J Cell Biol. 1981 Jun;24(2):320–323. [PubMed] [Google Scholar]
- Gonnella P. A., Nachmias V. T. Platelet activation and microfilament bundling. J Cell Biol. 1981 Apr;89(1):146–151. doi: 10.1083/jcb.89.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Höglund A. S., Karlsson R., Arro E., Fredriksson B. A., Lindberg U. Visualization of the peripheral weave of microfilaments in glia cells. J Muscle Res Cell Motil. 1980 Jun;1(2):127–146. doi: 10.1007/BF00711795. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., Fox J. E., Edwards H. H., Phillips D. R. Changes in the cytoskeletal structure of human platelets following thrombin activation. J Biol Chem. 1981 Jul 10;256(13):6927–6932. [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Mustard J. F., Packham M. A., Perry D. W., Reimers H. J., Cazenave J. P. Properties of washed human platelets. Thromb Haemost. 1977 Apr 30;37(2):291–308. [PubMed] [Google Scholar]
- Lengsfeld A. M., Löw I., Wieland T., Dancker P., Hasselbach W. Interaction of phalloidin with actin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2803–2807. doi: 10.1073/pnas.71.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. F., Fedorko M. E. Isolation of intact megakaryocytes from guinea pig femoral marrow. Successful harvest made possible with inhibitions of platelet aggregation; enrichment achieved with a two-step separation technique. J Cell Biol. 1976 Apr;69(1):159–172. doi: 10.1083/jcb.69.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus J. C., Choate J., Albrecht R. M. Platelet activation and cytoskeletal reorganization: high voltage electron microscopic examination of intact and Triton-extracted whole mounts. J Cell Biol. 1984 Jun;98(6):2019–2025. doi: 10.1083/jcb.98.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias V. T. Cytoskeleton of human platelets at rest and after spreading. J Cell Biol. 1980 Sep;86(3):795–802. doi: 10.1083/jcb.86.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Chaponnier C., Jeanrenaud B., Gabbiani G. Actin microfilaments, cell shape, and secretory processes in isolated rat hepatocytes. Effect of phalloidin and cytochalasin D. J Cell Biol. 1979 Jun;81(3):592–607. doi: 10.1083/jcb.81.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981 Dec;91(3 Pt 1):695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Onji T., Okamoto K., Matsusaka T., Taniguchi H., Shibata N. Reorganization of contractile elements in the platelet during clot retraction. J Ultrastruct Res. 1984 Oct;89(1):98–109. doi: 10.1016/s0022-5320(84)80027-1. [DOI] [PubMed] [Google Scholar]
- Wani M. C., Taylor H. L., Wall M. E., Coggon P., McPhail A. T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971 May 5;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- White J. G. Arrangements of actin filaments in the cytoskeleton of human platelets. Am J Pathol. 1984 Nov;117(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Burris S. M. Morphometry of platelet internal contraction. Am J Pathol. 1984 Jun;115(3):412–417. [PMC free article] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G., Gerrard J. M. Interaction of microtubules and microfilaments in platelet contractile physiology. Methods Achiev Exp Pathol. 1979;9:1–39. [PubMed] [Google Scholar]
- White J. G., Krumwiede M., Sauk J. J. Microtubule reassembly in surface-activated platelets. Blood. 1985 Jun;65(6):1494–1503. [PubMed] [Google Scholar]
- White J. G., Rao G. H. Influence of a microtubule stabilizing agent on platelet structural physiology. Am J Pathol. 1983 Aug;112(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Rao G. H. Influence of a microtubule stabilizing agent on platelet structural physiology. Am J Pathol. 1983 Aug;112(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Sauk J. J. Microtubule coils in spread blood platelets. Blood. 1984 Aug;64(2):470–478. [PubMed] [Google Scholar]
- White J. G. The submembrane filaments of blood platelets. Am J Pathol. 1969 Aug;56(2):267–277. [PMC free article] [PubMed] [Google Scholar]