Abstract
Bis(5-amidino-2-benzimidazolyl)methane (BABIM) is a synthetic aromatic amidine compound which has a number of important biochemical effects, including inhibition of a family of esteroproteases (trypsin, urokinase, plasmin) previously linked to the complex process of tumor invasion. Previous work has suggested that exogenous natural protease inhibitors can block invasion of tumor cells across basement membranes (BM) in vitro. The authors studied the effect of BABIM on the human cell line HT-1080 with the use of a quantitative in vitro amnion invasion assay system. They have verified the ability of these cells to grow in nude mice and metastasize via the lymphatics or blood vessels on the basis of the route of administration of the inoculum. Cells which were able to actively cross the entire BM were trapped on filters and counted by both brightfield microscopy and by beta scintillation counting of cells whose DNA was labeled with tritiated thymidine. In agreement with either counting technique, BABIM, at a concentration of 10(-4) M, significantly inhibited invasion (P less than 0.005) over the 7-day course of the experiments. Under these conditions, the inhibitor was nontoxic and did not alter the attachment of the cells to the amniotic membrane. Furthermore, a highly significant inhibition of invasion (P less than 0.001) was also demonstrated across a variation in molar concentration of BABIM of more than 2 orders of magnitude. Most remarkably, cells were initially inhibited in their ability to invade in the presence of between 10(-9) and 10(-3) M BABIM. Measurement of Type IV specific collagenase in media from these cells shows a significant inhibition of activity in the presence of BABIM. These results suggest two, not necessarily exclusive, alternative interpretations: first, that inhibition of the proteolytic steps along the pathway of activation of basement membrane degrading enzymes results in inhibition of invasion; second, that arginine directed esteroproteases may work in concert with cellular collagenolytic metalloproteinases in the process of invasion by human tumor cells through native matrix barriers.
Full text
PDF



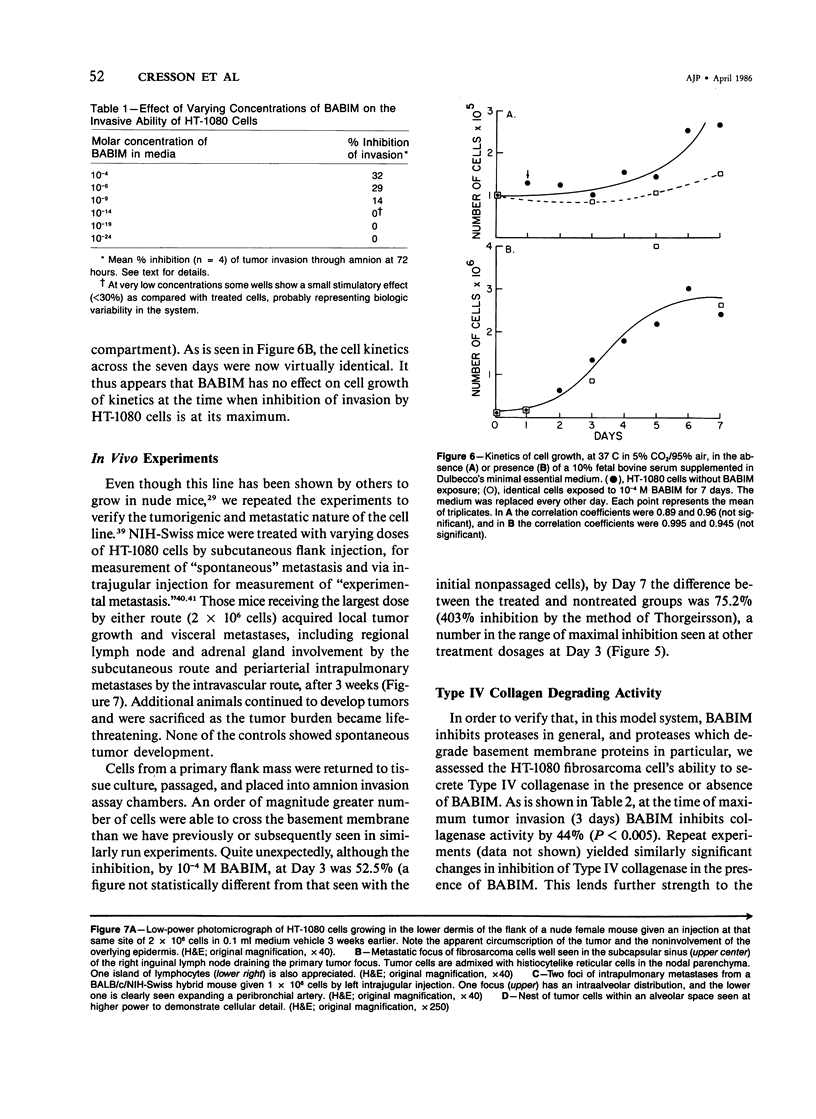
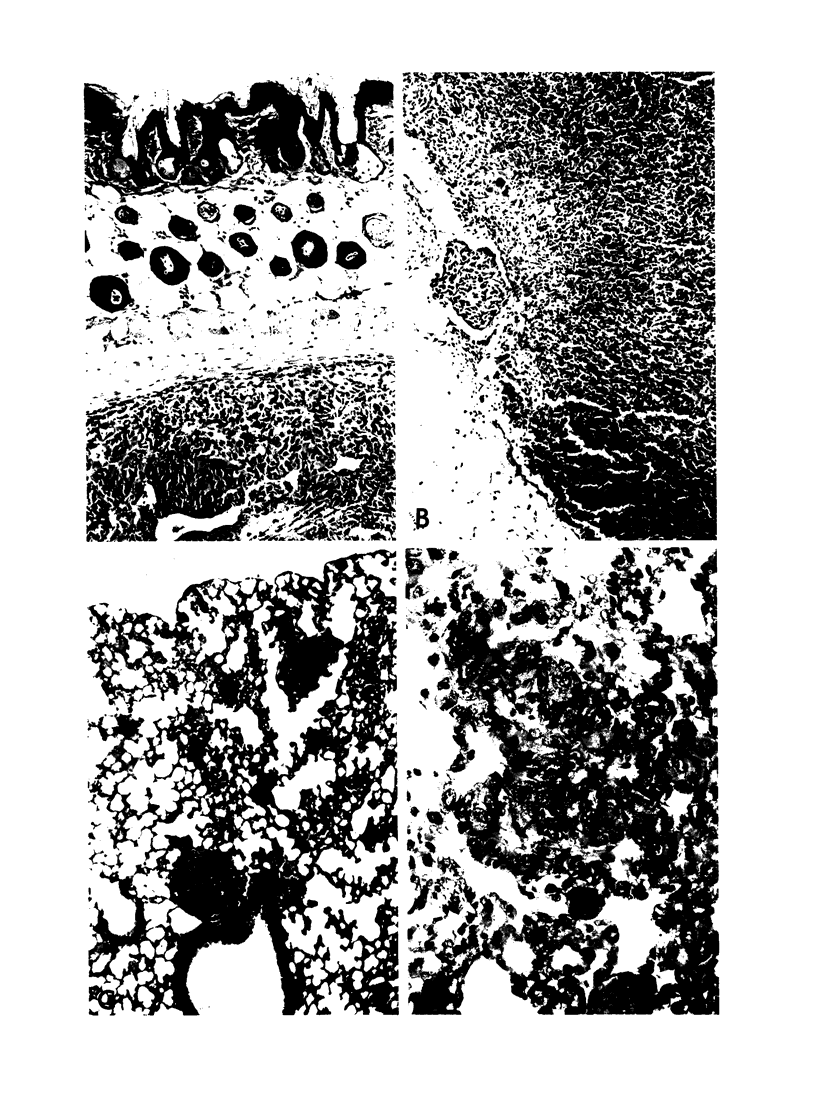



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Vaheri A., Krieg T., Timpl R. Biosynthesis of two subunits of type IV procollagen and of other basement membrane proteins by a human tumor cell line. Eur J Biochem. 1980 Aug;109(1):247–255. doi: 10.1111/j.1432-1033.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Baici A., Gyger-Marazzi M., Sträuli P. Extracellular cysteine proteinase and collagenase activities as a consequence of tumor-host interaction in the rabbit V2 carcinoma. Invasion Metastasis. 1984;4(1):13–27. [PubMed] [Google Scholar]
- Barrett J. C., Crawford B. D., Grady D. L., Hester L. D., Jones P. A., Benedict W. F., Ts'o P. O. Temporal acquistion of enhanced fibrinolytic activity by syrian hamster embryo cells following treatment with benzo(a)pyrene. Cancer Res. 1977 Oct;37(10):3815–3823. [PubMed] [Google Scholar]
- Barsky S. H., Baker A., Siegal G. P., Togo S., Liotta L. A. Use of anti-basement membrane antibodies to distinguish blood vessel capillaries from lymphatic capillaries. Am J Surg Pathol. 1983 Oct;7(7):667–677. doi: 10.1097/00000478-198310000-00007. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Bogenmann E., Jones P. A. Role of plasminogen in matrix breakdown by neoplastic cells. J Natl Cancer Inst. 1983 Dec;71(6):1177–1182. [PubMed] [Google Scholar]
- Camiolo S. M., Markus G., Englander L. S., Siuta M. R., Hobika G. H., Kohga S. Plasminogen activator content and secretion in explants of neoplastic and benign human prostate tissues. Cancer Res. 1984 Jan;44(1):311–318. [PubMed] [Google Scholar]
- Carlsen S. A., Ramshaw I. A., Warrington R. C. Involvement of plasminogen activator production with tumor metastasis in a rat model. Cancer Res. 1984 Jul;44(7):3012–3016. [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombi M., Barlati S., Magdelenat H., Fiszer-Szafarz B. Relationship between multiple forms of plasminogen activator in human breast tumors and plasma and the presence of metastases in lymph nodes. Cancer Res. 1984 Jul;44(7):2971–2975. [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Tidwell R. R. Enhancement of respiratory syncytial virus-induced cytopathology by trypsin, thrombin, and plasmin. Infect Immun. 1983 Apr;40(1):351–358. doi: 10.1128/iai.40.1.351-358.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geratz J. D., Shaver S. R., Tidwell R. R. Inhibitory effect of amidino-substituted heterocyclic compounds on the amidase activity of plasmin and of high and low molecular weight urokinase and on urokinase-induced plasminogen activation. Thromb Res. 1981 Oct 1;24(1-2):73–83. doi: 10.1016/0049-3848(81)90033-5. [DOI] [PubMed] [Google Scholar]
- Geratz J. D., Stevens F. M., Polakoski K. L., Parrish R. F., Tidwell R. R. Amidino-substituted aromatic heterocycles as probes of the specificity pocket of trypsin-like proteases. Arch Biochem Biophys. 1979 Oct 15;197(2):551–559. doi: 10.1016/0003-9861(79)90279-0. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Merchant B., Gelfand M. C., Vickers J., Steinberg A. D., Hansen C. T. The natural history and immunopathology of outbred athymic (nude) mice. Clin Immunol Immunopathol. 1975 Sep;4(3):324–340. doi: 10.1016/0090-1229(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Halaka A. N., Bunning R. A., Bird C. C., Gibson M., Reynolds J. J. Production of collagenase and inhibitor (TIMP) by intracranial tumors and dura in vitro. J Neurosurg. 1983 Sep;59(3):461–466. doi: 10.3171/jns.1983.59.3.0461. [DOI] [PubMed] [Google Scholar]
- Hall A., Marshall C. J., Spurr N. K., Weiss R. A. Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature. 1983 Jun 2;303(5916):396–400. doi: 10.1038/303396a0. [DOI] [PubMed] [Google Scholar]
- Haskill S., Kivinen S., Nelson K., Fowler W. C., Jr Detection of intratumor heterogeneity by simultaneous multiparameter flow cytometric analysis with enzyme and DNA markers. Cancer Res. 1983 Mar;43(3):1003–1009. [PubMed] [Google Scholar]
- Heisel M., Laug W. E., Jones P. A. Inhibition by bovine endothelial cells of degradation by HT-1080 fibrosarcoma cells of extracellular matrix proteins. J Natl Cancer Inst. 1983 Dec;71(6):1183–1187. [PubMed] [Google Scholar]
- Jones P. A., DeClerck Y. A. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980 Sep;40(9):3222–3227. [PubMed] [Google Scholar]
- Kozlowski J. M., Fidler I. J., Campbell D., Xu Z. L., Kaighn M. E., Hart I. R. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984 Aug;44(8):3522–3529. [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G. Selective degradation of basement membrane macromolecules by metastatic melanoma cells. J Natl Cancer Inst. 1984 Apr;72(4):889–899. [PubMed] [Google Scholar]
- Kuettner K. E., Soble L., Croxen R. L., Marczynska B., Hiti J., Harper E. Tumor cell collagenase and its inhibition by a cartilage-derived protease inhibitor. Science. 1977 May 6;196(4290):653–654. doi: 10.1126/science.193181. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Terranova V. P. Cleavage of laminin by thrombin and plasmin: alpha thrombin selectively cleaves the beta chain of laminin. Thromb Res. 1981 Mar 15;21(6):663–673. doi: 10.1016/0049-3848(81)90268-1. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Lee C. W., Morakis D. J. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980 Dec;11(2):141–152. doi: 10.1016/0304-3835(80)90105-6. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A., Thorgeirsson U. P., Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;1(4):277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor extracellular matrix. Lab Invest. 1982 Aug;47(2):112–113. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Mareel M., Kint J., Meyvisch C. Methods of study of the invasion of malignant C3H-mouse fibroblasts into embryonic chick heart in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979 May 4;30(1):95–111. doi: 10.1007/BF02889094. [DOI] [PubMed] [Google Scholar]
- Markus G., Camiolo S. M., Kohga S., Madeja J. M., Mittelman A. Plasminogen activator secretion of human tumors in short-term organ culture, including a comparison of primary and metastatic colon tumors. Cancer Res. 1983 Nov;43(11):5517–5525. [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Di Ferrante D., Di Ferrante N., Nicolson G. L. Heparan sulfate degradation: relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science. 1983 May 6;220(4597):611–613. doi: 10.1126/science.6220468. [DOI] [PubMed] [Google Scholar]
- Ng R., Kellen J. A. The role of plasminogen activators in metastasis. Med Hypotheses. 1983 Mar;10(3):291–293. doi: 10.1016/0306-9877(83)90116-0. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Paranjpe M., Engel L., Young N., Liotta L. A. Activation of human breast carcinoma collagenase through plasminogen activator. Life Sci. 1980 Apr 14;26(15):1223–1231. doi: 10.1016/0024-3205(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Roberts J. A. Cathepsin B-like enzymes. Subcellular distribution and properties in neoplastic and control cells from human ectocervix. J Biol Chem. 1981 Aug 25;256(16):8536–8544. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Nicolson G. L. Experimental systems for analysis of the surface properties of metastatic tumor cells. Biomembranes. 1983;11:341–364. [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Russo R. G., Liotta L. A., Thorgeirsson U., Brundage R., Schiffmann E. Polymorphonuclear leukocyte migration through human amnion membrane. J Cell Biol. 1981 Nov;91(2 Pt 1):459–467. doi: 10.1083/jcb.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Keski-Oja J., Turpeenniemi-Hujanen T., Tryggvason K. Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells--role in metastasis. Int J Cancer. 1982 Nov 15;30(5):669–673. doi: 10.1002/ijc.2910300520. [DOI] [PubMed] [Google Scholar]
- Siegal G. P., Thorgeirsson U. P., Russo R. G., Wallace D. M., Liotta L. A., Berger S. L. Interferon enhancement of the invasive capacity of Ewing sarcoma cells in vitro. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4064–4068. doi: 10.1073/pnas.79.13.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane B. F., Dunn J. R., Honn K. V. Lysosomal cathepsin B: correlation with metastatic potential. Science. 1981 Jun 5;212(4499):1151–1153. doi: 10.1126/science.7233209. [DOI] [PubMed] [Google Scholar]
- Sträuli P., Haemmerli G. The role of cancer cell motility in invasion. Cancer Metastasis Rev. 1984;3(2):127–141. doi: 10.1007/BF00047660. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Liotta L. A., Kalebic T., Margulies I. M., Thomas K., Rios-Candelore M., Russo R. G. Effect of natural protease inhibitors and a chemoattractant on tumor cell invasion in vitro. J Natl Cancer Inst. 1982 Nov;69(5):1049–1054. [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Neckers L. M., Johnson D. W., Liotta L. A. Protein synthesis but not DNA synthesis is required for tumor cell invasion in vitro. Invasion Metastasis. 1984;4(2):73–83. [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Clyde W. A., Jr, Rosenthal K. U., Dubovi E. J. Suppression of respiratory syncytial virus infection in cotton rats by bis(5-amidino-2-benzimidazolyl)methane. Antimicrob Agents Chemother. 1984 Oct;26(4):591–593. doi: 10.1128/aac.26.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dann O., Volz G., Zeh D., Loewe H. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole-like ring. Inhibitors of arginine-specific esteroproteases. J Med Chem. 1978 Jul;21(7):613–623. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dubovi E. J. Aromatic amidines: comparison of their ability to block respiratory syncytial virus induced cell fusion and to inhibit plasmin, urokinase, thrombin, and trypsin. J Med Chem. 1983 Feb;26(2):294–298. doi: 10.1021/jm00356a036. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Tidwell R. R., Rector T. M., Geratz J. D. Effect of synthetic protease inhibitors of the amidine type on cell injury by Rickettsia rickettsii. Antimicrob Agents Chemother. 1984 May;25(5):582–585. doi: 10.1128/aac.25.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Aggeler J. Proteases induce secretion of collagenase and plasminogen activator by fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1839–1843. doi: 10.1073/pnas.75.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Roberts D. R., Evanson J. M. Small molecular weight beta 1 serum protein which specifically inhibits human collagenases. Nature. 1976 May 27;261(5558):325–327. doi: 10.1038/261325a0. [DOI] [PubMed] [Google Scholar]
- Yang N. S., Kirkland W., Jorgensen T., Furmanski P. Absence of fibronectin and presence of plasminogen activator in both normal and malignant human mammary epithelial cells in culture. J Cell Biol. 1980 Jan;84(1):120–130. doi: 10.1083/jcb.84.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]