Abstract
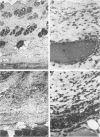
Killed Escherichia coli organisms injected intradermally into rabbits induced significant neutropenia and provoked a rapid rise in body temperature. Both the magnitude and the duration of the neutropenia were dose-dependent. After recovery from neutropenia, the rabbits became refractory to its redevelopment when subsequently given an equivalent dose of E coli. The influence of neutropenia and the subsequent refractory period on the rate of polymorphonuclear leukocyte (PMN) emigration into inflammatory sites was examined. Killed E coli organisms (6 X 10(8) per site) were injected into two groups of 20 intradermal sites in each rabbit. The first group (Group F) preceded the second (Group S) by 6 hours. The kinetics of PMN emigration, quantitated with 51Cr-labeled cells, differed in the two groups. In Group S sites an intense PMN influx was measured at 0-4 hours, and subsequently the extent of PMN emigration rapidly declined. In Group F sites a minute PMN influx was detected during the first 4 hours, coinciding with a marked neutropenia. The maximal PMN influx into Group F sites was measured between 6 and 10 hours. Microscopic sections at 4 hours showed a scanty PMN infiltrate and numerous bacteria in the dermis of Group F sites, while extensive phagocytosis of bacteria by PMNs was apparent in Group S sites. By comparing the extent of bacterial phagocytosis in 4-hour-old sites with the magnitudes of PMN emigration between 6 and 10 hours in both groups, we concluded that the phagocytic elimination of killed E coli was not a major mechanism regulating the cessation of local PMN emigration. Instead, we propose that tachyphylaxis or desensitization of sites to inflammatory factors released from E coli is the responsible mechanism.
Full text
PDF

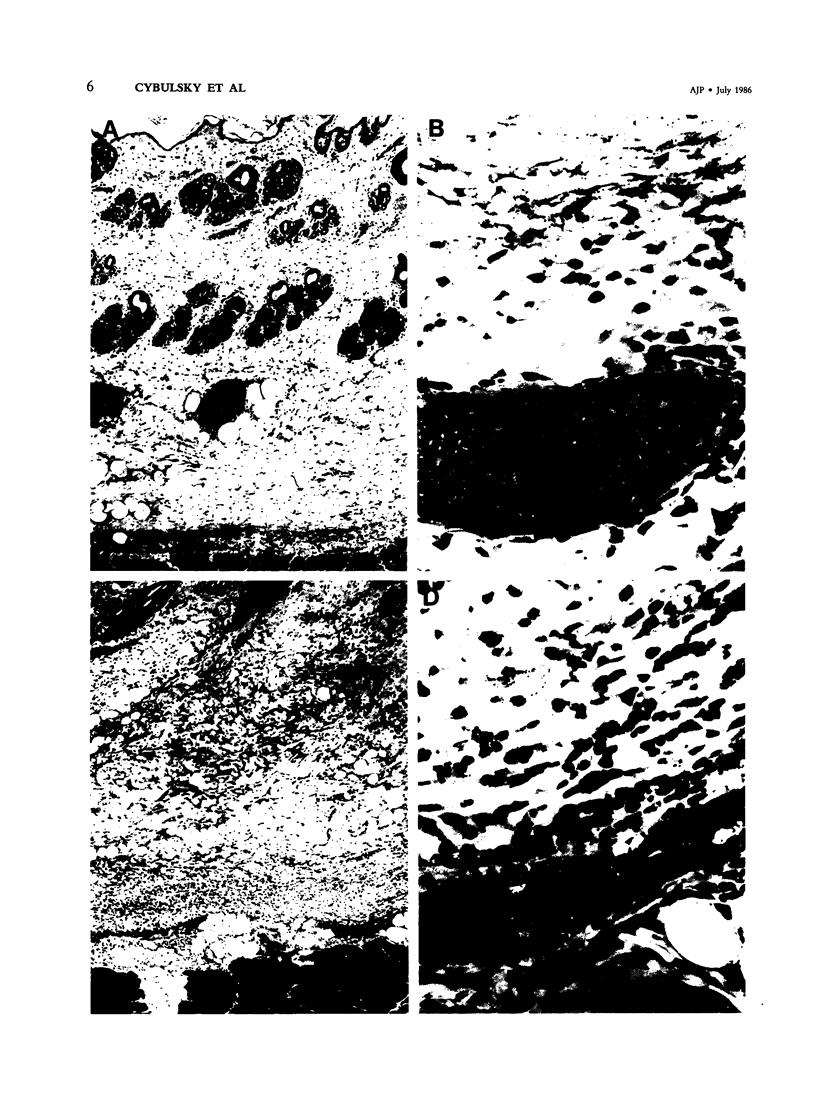
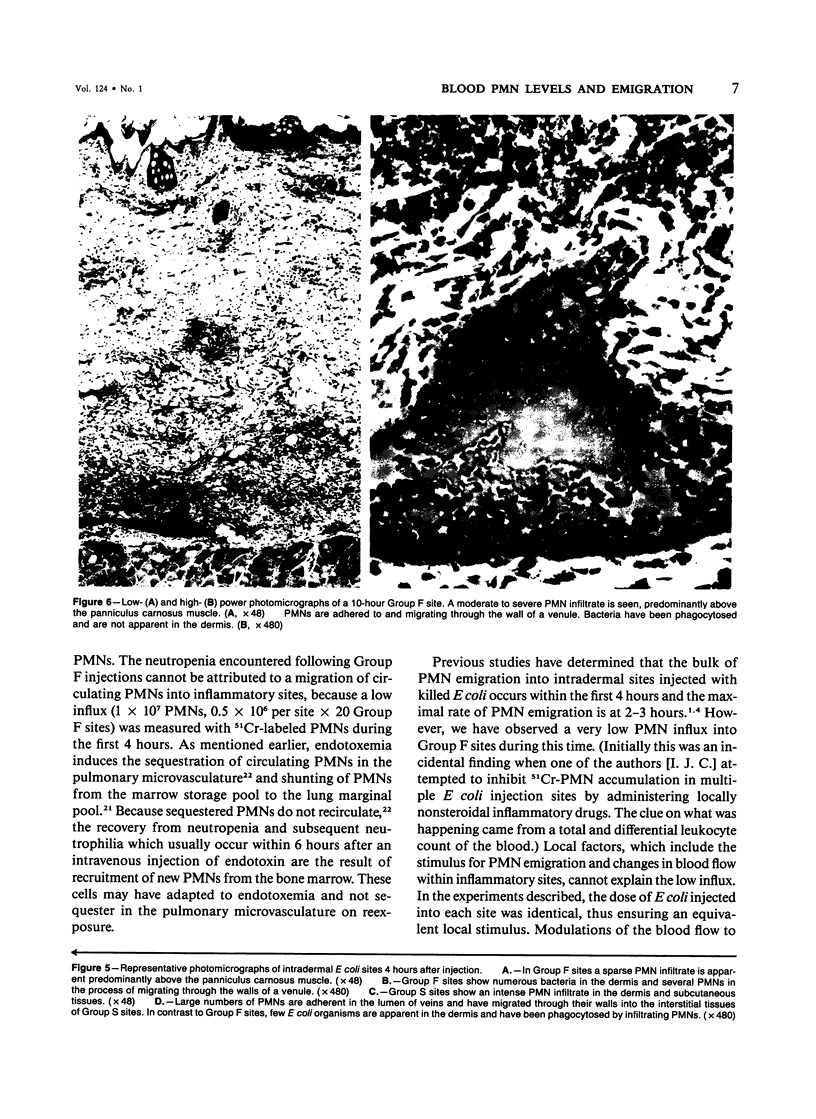


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baue A. E., Chaudry I. H. Prevention of multiple systems failure. Surg Clin North Am. 1980 Oct;60(5):1167–1178. doi: 10.1016/s0039-6109(16)42240-1. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz I. G. Margination and emigration of leucocytes. Surv Synth Pathol Res. 1985;4(1):44–68. doi: 10.1159/000156964. [DOI] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Chemotactic factor-specific desensitization of skin to infiltration by polymorphonuclear leukocytes. Immunol Lett. 1984;8(2):83–87. doi: 10.1016/0165-2478(84)90055-5. [DOI] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Desensitization of acute inflammatory lesions to chemotaxins and endotoxin. J Immunol. 1984 Oct;133(4):2163–2168. [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Cybulsky M. I., Movat H. Z. Experimental bacterial pneumonia in rabbits: polymorphonuclear leukocyte margination and sequestration in rabbit lungs and quantitation and kinetics of 51Cr-labeled polymorphonuclear leukocytes in E. coli-induced lung lesions. Exp Lung Res. 1982 Dec;4(1):47–66. doi: 10.3109/01902148209039249. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W. Egestion of degraded meningococci by polymorphonuclear leukocytes. J Bacteriol. 1976 Jan;125(1):258–266. doi: 10.1128/jb.125.1.258-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Gilbertsen R. B., Carter G. W., Quinn D. J. Effects of F-met-leu-phe and zymosan-activated serum on rat neutrophils in vivo. J Reticuloendothel Soc. 1980 May;27(5):485–494. [PubMed] [Google Scholar]
- Hay J. B., Johnston M. G., Hobbs B. B., Movat H. Z. The use of radioactive microspheres to quantitate hyperemia in dermal inflammatory sites. Proc Soc Exp Biol Med. 1975 Dec;150(3):641–644. doi: 10.3181/00379727-150-39096. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S., Bortolussi R. Effect of immune serum or polymyxin B on Escherichia coli-induced inflammation and vascular injury. Infect Immun. 1982 May;36(2):548–557. doi: 10.1128/iai.36.2.548-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S. Role for endotoxin in the leukocyte infiltration accompanying Escherichia coli inflammation. Infect Immun. 1982 May;36(2):558–566. doi: 10.1128/iai.36.2.558-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C. Effect of vasoactive agents on polymorphonuclear leukocyte emigration in vivo. Lab Invest. 1981 Sep;45(3):234–240. [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The effect of vasodilator prostaglandins on polymorphonuclear leukocyte infiltration and vascular injury. Am J Pathol. 1982 Jun;107(3):300–309. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Issekutz A. C., Ripley M., Rochon Y., Pi-Jimenez E., Wright B. A role for hemolysin in Escherichia coli-induced inflammation in granulocytopenic rabbits. J Infect Dis. 1984 Dec;150(6):925–934. doi: 10.1093/infdis/150.6.925. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of monocyte migration into acute inflammatory tissue. Am J Pathol. 1981 Apr;103(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Jeynes B. J., Issekutz A. C., Issekutz T. B., Movat H. Z. Quantitation of platelets in the microcirculation. Measurement of indium-111 in microthrombi induced in rabbits by inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Dec;165(3):445–452. doi: 10.3181/00379727-165-41002. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Burrowes C. E., Movat H. Z. The quantitation of hemorrhage in the skin. Measurement of hemorrhage in the microcirculation in inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Jan;163(1):126–131. doi: 10.3181/00379727-163-40733. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Movat H. Z. Kinetics of acute inflammation induced by Escherichia coli in rabbits. II. The effect of hyperimmunization, complement depletion, and depletion of leukocytes. Am J Pathol. 1983 Jan;110(1):13–29. [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Sinanan M., Maier R. V., Carrico C. J. Laparotomy for intra-abdominal sepsis in patients in an intensive care unit. Arch Surg. 1984 Jun;119(6):652–658. doi: 10.1001/archsurg.1984.01390180020004. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K. Microbial interactions with neutrophils. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S806–S822. doi: 10.1093/clinids/5.supplement_4.s806. [DOI] [PubMed] [Google Scholar]
- Tobin M. J., Grenvik A. Nosocomial lung infection and its diagnosis. Crit Care Med. 1984 Mar;12(3):191–199. doi: 10.1097/00003246-198403000-00008. [DOI] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Hill H. R. Inflammatory mediators stimulate granulocyte adherence to cultured human endothelial cells. Thromb Res. 1984 Jul 15;35(2):203–217. doi: 10.1016/0049-3848(84)90215-9. [DOI] [PubMed] [Google Scholar]