Abstract
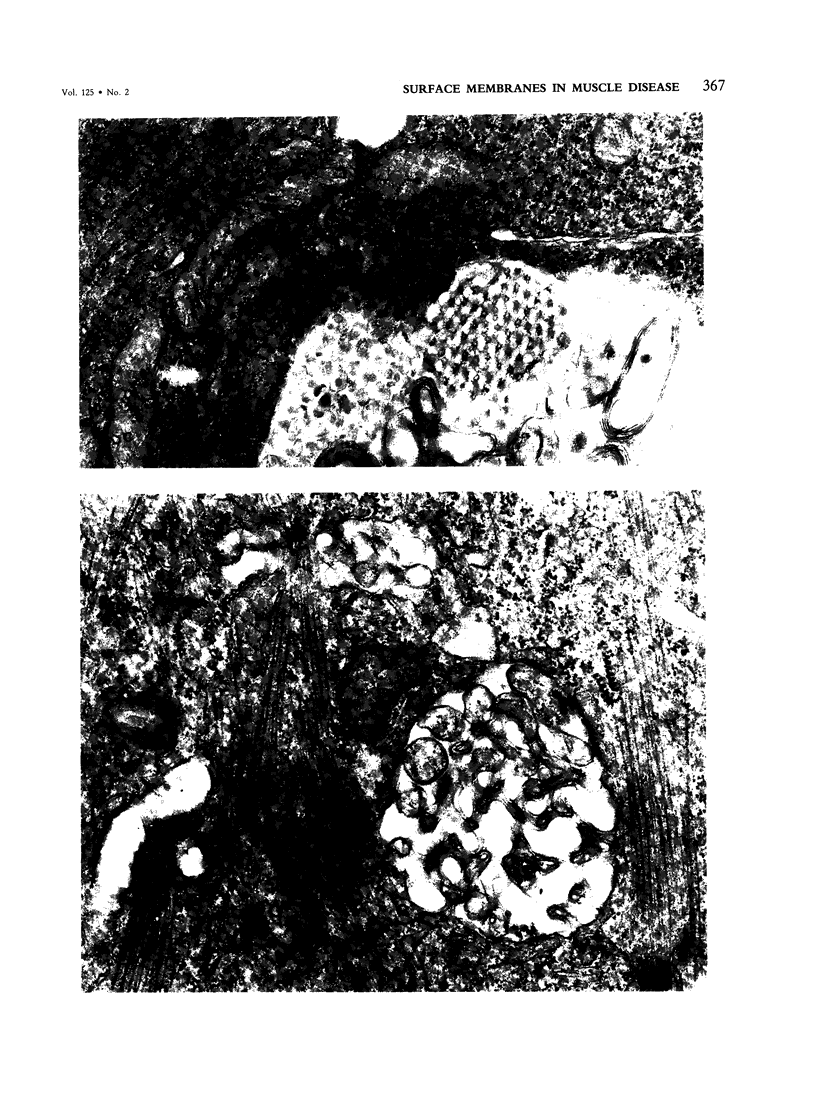
A surface-connected intracytoplasmic membranous (SCIM) network proliferates in skeletal muscle diseases and in myotubes grown in vitro. The authors observed frequent occurrence of "coated" microdomains in the form of budding vesicles in the proliferated components of this network and suspected a potential role the proliferated membranes might have in the endocytosis of molecules into myotubes undergoing repair or regeneration. Five-day-old myotubes in culture were incubated at 37 C and between 2 and 4 C with two tracers, Lucifer yellow and ferritin, both known to enter other types of cells via a fluid-phase endocytotic pathway. The differential penetration of Lucifer yellow at 37 C and below 2-4 C was examined by fluorescence microscopy and by electron microscopy. Lucifer yellow was rendered electron-opaque by photoreacting it with an intense light in the presence of DAB. Ferritin penetration at 37 C and between 2 and 4 C was compared and quantitated ultrastructurally. The authors found that endocytosis of the tracers into myotubes and eventually into lysosomes took place after the tracers had diffused into the lumen of the proliferated SCIM network. These processes were inhibited below 4 C. This finding, coupled with the presence of "coated" microdomains in the proliferated membranes, led us to suspect that the SCIM network may have a role in membrane turnover of metabolically active diseased muscle cells undergoing regeneration.
Full text
PDF



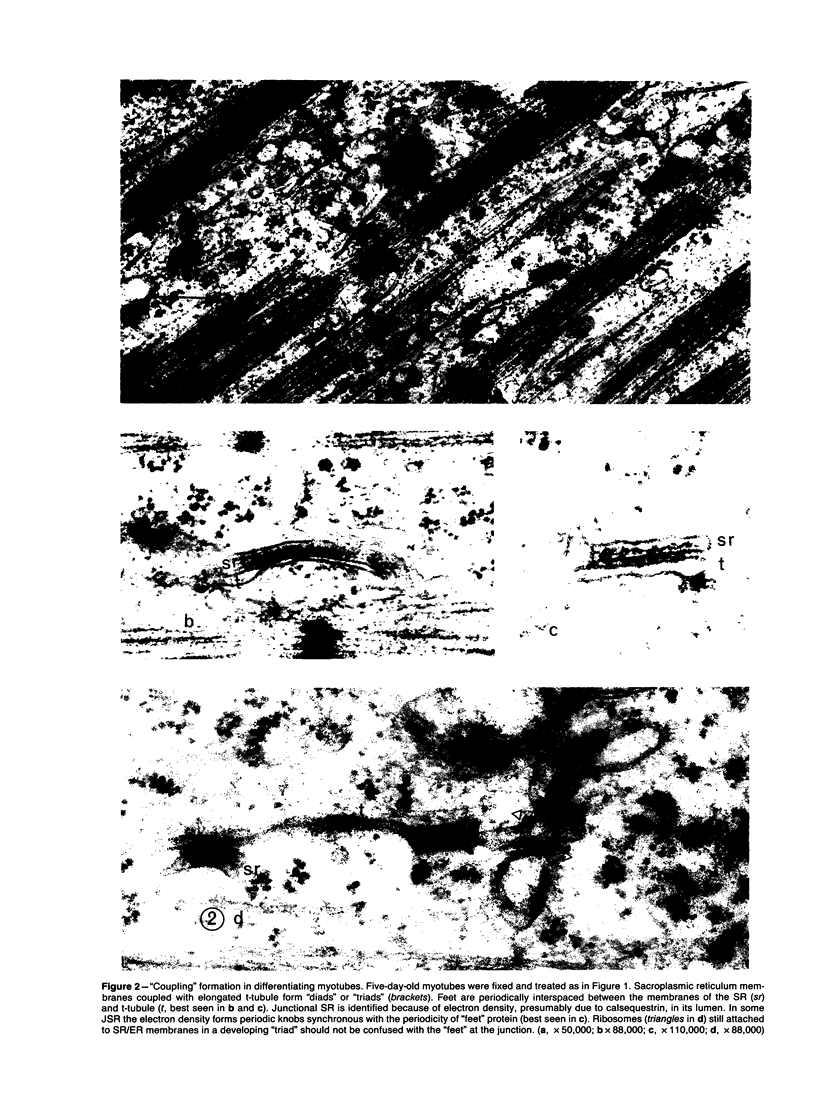
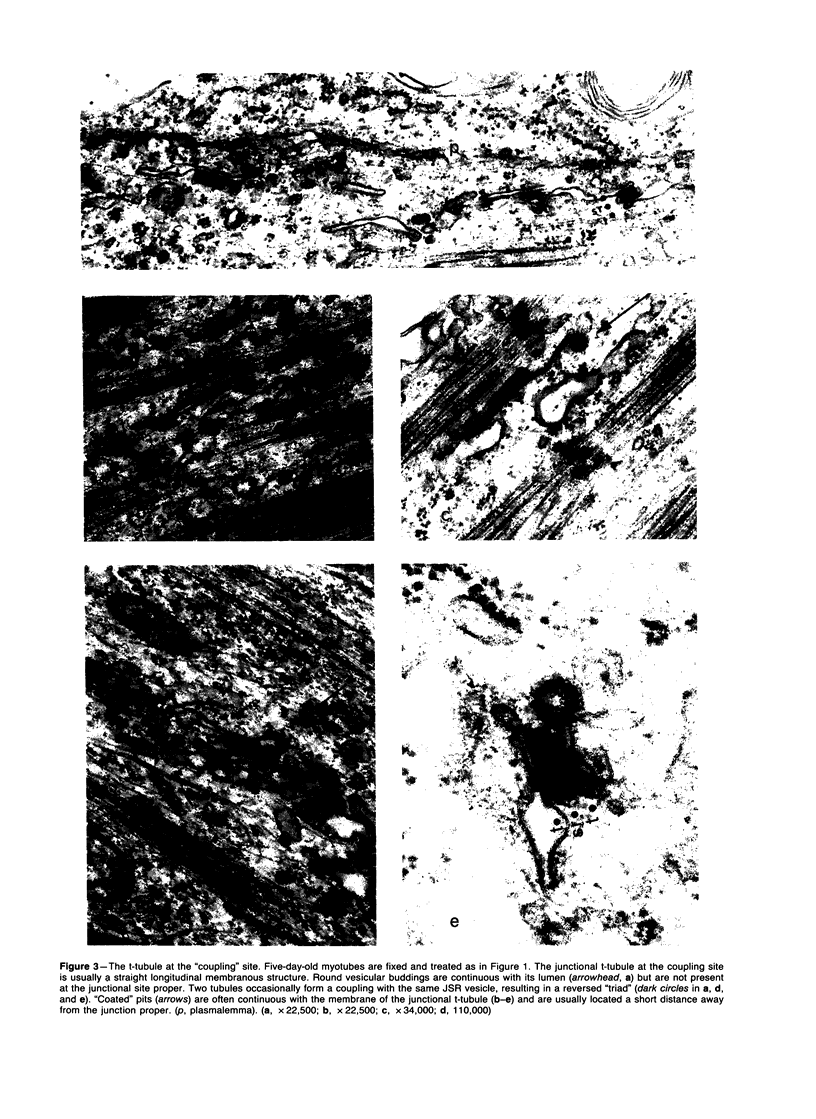
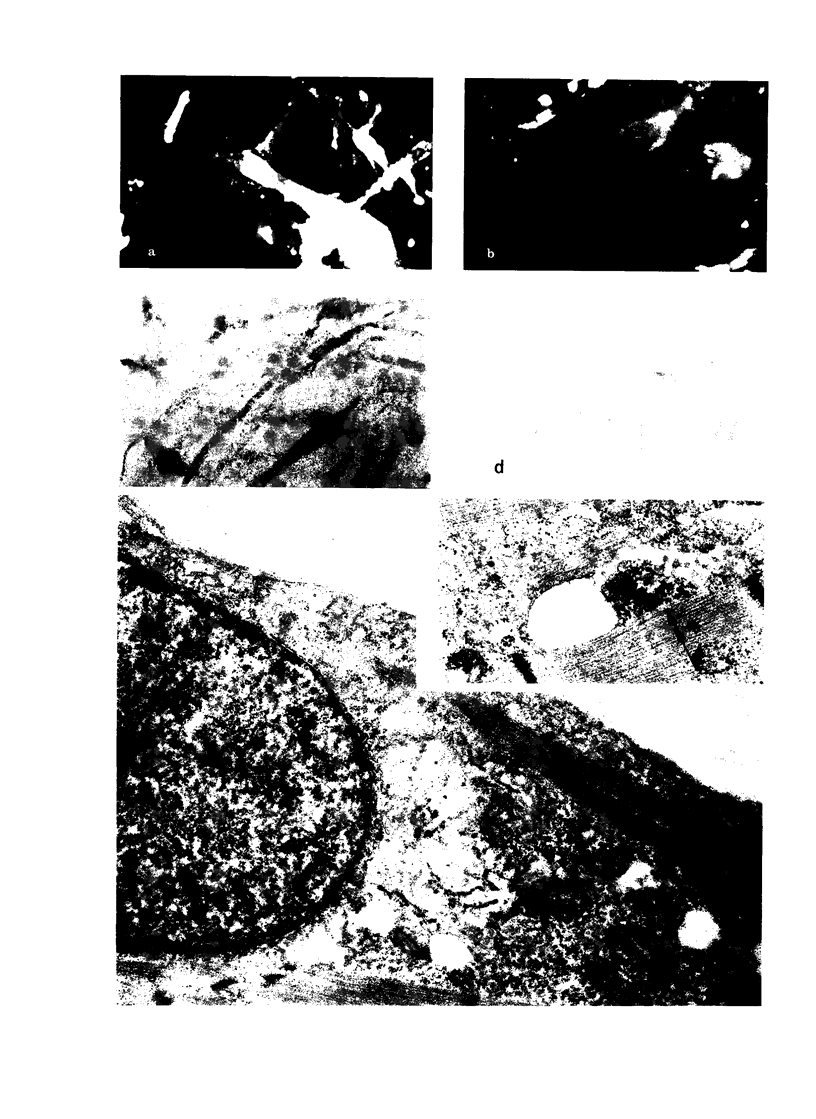



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn Z. A., Steinman R. M. Phagocytosis and fluid-phase pinocytosis. Ciba Found Symp. 1982;(92):15–34. doi: 10.1002/9780470720745.ch2. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Intracellular membrane traffic: pathways, carriers, and sorting devices. Methods Enzymol. 1983;98:1–13. doi: 10.1016/0076-6879(83)98134-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Formation of elaborate networks of T-system tubules in cultured skeletal muscle with special reference to the T-system formation. J Cell Biol. 1968 Jul;38(1):51–66. doi: 10.1083/jcb.38.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Campbell K. P., MacLennan D. H. Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1983 Nov;97(5 Pt 1):1573–1581. doi: 10.1083/jcb.97.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libelius R., Jirmanová I., Lundquist I., Thesleff S., Barnard E. A. T-tubule endocytosis in dystrophic chicken muscle and its relation to muscle fiber degeneration. Acta Neuropathol. 1979 Oct;48(1):31–38. doi: 10.1007/BF00691788. [DOI] [PubMed] [Google Scholar]
- Malouf N. N., Sommer J. R. Chicken dystrophy. The geometry of the transverse tubules. Am J Pathol. 1976 Aug;84(2):299–316. [PMC free article] [PubMed] [Google Scholar]
- Maranto A. R. Neuronal mapping: a photooxidation reaction makes Lucifer yellow useful for electron microscopy. Science. 1982 Sep 3;217(4563):953–955. doi: 10.1126/science.7112109. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975 Apr 21;389(1):51–68. doi: 10.1016/0005-2736(75)90385-5. [DOI] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Cantini M., Sartore S. T-system formation in cultured rat skeletal tissue. Tissue Cell. 1977;9(3):437–446. doi: 10.1016/0040-8166(77)90004-0. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Margreth A. Coordinated development of the sarcoplasmic reticulum and T system during postnatal differentiation of rat skeletal muscle. J Cell Biol. 1969 Jun;41(3):855–875. doi: 10.1083/jcb.41.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotland D. L. An electron microscopic investigation of myotonic dystrophy. J Neuropathol Exp Neurol. 1970 Apr;29(2):241–253. [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]