Abstract
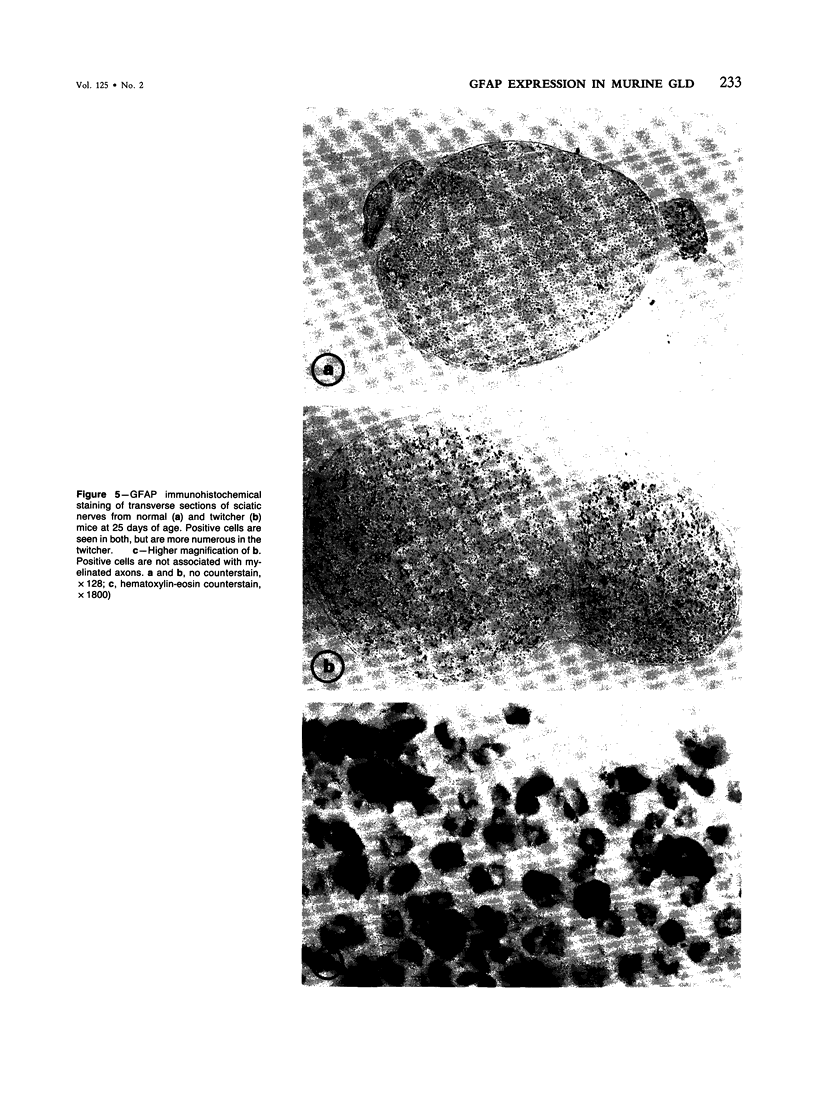
The expression of glial fibrillary acidic protein (GFAP) was found to be markedly enhanced immunohistochemically and biochemically both in the central (CNS) and peripheral (PNS) nervous systems of the twitcher mutant, an authentic murine model of human globoid cell leukodystrophy. The astrocytes in the CNS, the unmyelinated Schwann cells in the sciatic nerve, and the satellite cells in the trigeminal ganglion stained heavily with anti-GFAP antiserum. These changes in GFAP expression occurred shortly before the initiation of demyelination and coincided chronologically and topographically with infiltration of macrophages, suggesting that the same or closely related factors trigger the infiltration of macrophages and activate expression of GFAP. Cytoskeletal protein preparations showed increases in GFAP as well as in vimentin in the brainstem, spinal cord, and sciatic nerve. These results demonstrate that at least two types of peripheral glia (the unmyelinated Schwann cell and the satellite cell), in addition to the astrocyte, respond to some pathologic stimuli with an increased expression of GFAP. However, two other GFAP-positive structures, the Bergmann and radial glia, showed no significant changes in their immunostaining.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaducci L., Forno K. I., Eng L. F. Glial fibrillary acidic protein in cryogenic lesions of the rat brain. Neurosci Lett. 1981 Jan 1;21(1):27–32. doi: 10.1016/0304-3940(81)90052-5. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974 Jan 1;153(1):27–38. doi: 10.1002/cne.901530104. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Astrocyte-specific protein and radial glia in the cerebral cortex of newborn rat. Nature. 1974 Nov 1;252(5478):55–56. doi: 10.1038/252055a0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Differentiation of astrocytes in the cerebellar cortex and the pyramidal tracts of the newborn rat. An immunofluorescence study with antibodies to a protein specific to astrocytes. Brain Res. 1973 Jan 30;49(2):393–402. doi: 10.1016/0006-8993(73)90430-7. [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Liem R. K., Mason C. A. Development of cerebellar astroglia: transitions in form and cytoskeletal content. Dev Biol. 1984 Mar;102(1):248–259. doi: 10.1016/0012-1606(84)90189-1. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Nixon R. A., Strocchi P., Marotta C. A. Characterization and comparison of neurofilament proteins from rat and mouse CNS. J Neurochem. 1981 Jan;36(1):143–153. doi: 10.1111/j.1471-4159.1981.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Goldman J. E. Regulation of glial fibrillary acidic protein (GFAP) expression in CNS development and in pathological states. J Neuroimmunol. 1985 Jun;8(4-6):283–292. doi: 10.1016/s0165-5728(85)80067-9. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Goldman J. E. Synthesis and turnover of cytoskeletal proteins in cultured astrocytes. J Neurochem. 1984 Jan;42(1):166–174. doi: 10.1111/j.1471-4159.1984.tb09713.x. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Korey B., Norton W. T. Intermediate filaments from bovine, rat, and human CNS: mapping analysis of the major proteins. J Neurochem. 1980 May;34(5):1149–1159. doi: 10.1111/j.1471-4159.1980.tb09954.x. [DOI] [PubMed] [Google Scholar]
- Chiu F. C., Norton W. T., Fields K. L. The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin. J Neurochem. 1981 Jul;37(1):147–155. doi: 10.1111/j.1471-4159.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Kim R. C. Expression of glial fibrillary acidic protein by immature oligodendroglia and its implications. J Neuroimmunol. 1985 Jun;8(4-6):215–235. doi: 10.1016/s0165-5728(85)80064-3. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Kim R. C. Expression of glial fibrillary acidic protein in immature oligodendroglia. Science. 1984 Jan 27;223(4634):407–409. doi: 10.1126/science.6197755. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Lapham L. W. Radial glia in the human fetal cerebrum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res. 1978 Jun 16;148(2):295–311. doi: 10.1016/0006-8993(78)90721-7. [DOI] [PubMed] [Google Scholar]
- Dahl D., Chi N. H., Miles L. E., Nguyen B. T., Bignami A. Glial fibrillary acidic (GFA) protein in Schwann cells: fact or artifact? J Histochem Cytochem. 1982 Sep;30(9):912–918. doi: 10.1177/30.9.6182187. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Eicher E. M., Jacobs J. M., Scaravilli F., Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980 Sep;103(3):695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- Dupouey P., Lucas C. V., Gomes D., Jacque C. Immunohistochemical localization of the myelin basic protein and of the glial fibrillary acidic protein: comparative study in normal, quaking and jimpy mice. J Neurosci Res. 1980;5(5):387–398. doi: 10.1002/jnr.490050504. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Rubinstein L. J. Contribution of immunohistochemistry to diagnostic problems of human cerebral tumors. J Histochem Cytochem. 1978 Jul;26(7):513–522. doi: 10.1177/26.7.357640. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Fields K. L., Yen S. H. A subset of Schwann cells in peripheral nerves contain a 50-kDa protein antigenically related to astrocyte intermediate filaments. J Neuroimmunol. 1985 Jun;8(4-6):311–330. doi: 10.1016/s0165-5728(85)80070-9. [DOI] [PubMed] [Google Scholar]
- Gard A. L., White F. P., Dutton G. R. Extra-neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of rat liver. J Neuroimmunol. 1985 Jun;8(4-6):359–375. doi: 10.1016/s0165-5728(85)80073-4. [DOI] [PubMed] [Google Scholar]
- Hatfield J. S., Skoff R. P., Maisel H., Eng L., Bigner D. D. The lens epithelium contains glial fibrillary acidic protein (GFAP). J Neuroimmunol. 1985 Jun;8(4-6):347–357. doi: 10.1016/s0165-5728(85)80072-2. [DOI] [PubMed] [Google Scholar]
- Hatfield J. S., Skoff R. P., Maisel H., Eng L. Glial fibrillary acidic protein is localized in the lens epithelium. J Cell Biol. 1984 May;98(5):1895–1898. doi: 10.1083/jcb.98.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Igisu H., Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984 May 18;224(4650):753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci. 1983 Nov;3(11):2206–2218. doi: 10.1523/JNEUROSCI.03-11-02206.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Glial fibrillary acidic polypeptides in peripheral glia. Molecular weight, heterogeneity and distribution. J Neuroimmunol. 1985 Jun;8(4-6):377–393. doi: 10.1016/s0165-5728(85)80074-6. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Nonmyelin-forming Schwann cells coexpress surface proteins and intermediate filaments not found in myelin-forming cells: a study of Ran-2, A5E3 antigen and glial fibrillary acidic protein. J Neurocytol. 1984 Dec;13(6):923–934. doi: 10.1007/BF01148594. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Katayama M., Bourque E., Suzuki K., Suzuki K. The twitcher mouse: positive immunohistochemical staining of globoid cells with monoclonal antibody against Mac-1 antigen. Brain Res. 1985 May;352(1):49–54. doi: 10.1016/0165-3806(85)90086-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nagara H., Suzuki K., Suzuki K. The twitcher mouse: determination of genetic status by galactosylceramidase assays on clipped tail. Biochem Med. 1982 Feb;27(1):8–14. doi: 10.1016/0006-2944(82)90003-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Yamanaka T., Jacobs J. M., Teixeira F., Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease). Brain Res. 1980 Dec 8;202(2):479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- Latov N., Nilaver G., Zimmerman E. A., Johnson W. G., Silverman A. J., Defendini R., Cote L. Fibrillary astrocytes proliferate in response to brain injury: a study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev Biol. 1979 Oct;72(2):381–384. doi: 10.1016/0012-1606(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Lee V., Yen S. H., Shelanski M. L. Biochemical correlates of astrocytic proliferation in the mutant Staggerer mouse. Brain Res. 1977 Jun 10;128(2):389–392. doi: 10.1016/0006-8993(77)91007-1. [DOI] [PubMed] [Google Scholar]
- Levitt P., Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980 Oct 1;193(3):815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Ludwin S. K., Kosek J. C., Eng L. F. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976 Jan 15;165(2):197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K., Fujishiro M., Kohsaka S., Okano H., Takamatsu K., Tsukada Y. Disorders in myelination in the twitcher mutant: immunohistochemical and biochemical studies. Neurochem Res. 1985 Aug;10(8):1129–1141. doi: 10.1007/BF00965887. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K., Nagaike K., Aoki E., Tsukada Y. Biochemical and immunohistochemical studies on dysmyelination of quaking mutant mice in vivo and in vitro. Brain Res. 1979 Nov 16;177(2):287–299. doi: 10.1016/0006-8993(79)90780-7. [DOI] [PubMed] [Google Scholar]
- Nagara H., Kobayashi T., Suzuki K., Suzuki K. The twitcher mouse: normal pattern of early myelination in the spinal cord. Brain Res. 1982 Jul 29;244(2):289–294. doi: 10.1016/0006-8993(82)90087-7. [DOI] [PubMed] [Google Scholar]
- Pixley S. K., de Vellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 1984 Aug;317(2):201–209. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- Roessmann U., Velasco M. E., Sindely S. D., Gambetti P. Glial fibrillary acidic protein (GFAP) in ependymal cells during development. An immunocytochemical study. Brain Res. 1980 Oct 27;200(1):13–21. doi: 10.1016/0006-8993(80)91090-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W., Prensky A. L. Quantitative and qualitative study of sural nerve biopsies in Krabbe's disease. Acta Neuropathol. 1972;20(1):55–66. doi: 10.1007/BF00687902. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 1981 Aug;90(2):435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Salazar F. J., Abraham C., Kosik K. S. Huntington's disease: changes in striatal proteins reflect astrocytic gliosis. Brain Res. 1982 Aug 5;245(1):117–125. doi: 10.1016/0006-8993(82)90344-4. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Somera F. P., Eng L. F. Immunocytochemical staining for glial fibrillary acidic protein and the metabolism of cytoskeletal proteins in experimental allergic encephalomyelitis. Brain Res. 1983 Apr 4;264(2):241–253. doi: 10.1016/0006-8993(83)90822-3. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Somera F. P., Swanson K., Eng L. F. Glial fibrillary acidic protein in acute and chronic relapsing experimental allergic encephalomyelitis (EAE). Prog Clin Biol Res. 1984;146:139–144. [PubMed] [Google Scholar]
- Takahashi H., Igisu H., Suzuki K., Suzuki K. The twitcher mouse: an ultrastructural study on the oligodendroglia. Acta Neuropathol. 1983;59(3):159–166. doi: 10.1007/BF00703199. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Suzuki K. Demyelination in the spinal cord of murine globoid cell leukodystrophy (the twitcher mouse). Acta Neuropathol. 1984;62(4):298–308. doi: 10.1007/BF00687612. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J., Kohler R., Heitz P. U., Probst A. Immunocytochemical investigations of some human leukodystrophies. Acta Neuropathol. 1983;60(3-4):199–206. doi: 10.1007/BF00691867. [DOI] [PubMed] [Google Scholar]
- Uyeda C. T., Eng L. F., Bignami A. Immunological study of the glial fibrillary acidic protein. Brain Res. 1972 Feb 11;37(1):81–89. doi: 10.1016/0006-8993(72)90347-2. [DOI] [PubMed] [Google Scholar]
- Woodhams P. L., Bascó E., Hajós F., Csillág A., Balázs R. Radial glia in the developing mouse cerebral cortex and hippocampus. Anat Embryol (Berl) 1981;163(3):331–343. doi: 10.1007/BF00315709. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry F., Picart R., Jacque C., Tixier-Vidal A. Glial fibrillary acidic protein. A cellular marker of tanycytes in the mouse hypothalamus. Dev Neurosci. 1981;4(6):457–460. doi: 10.1159/000112813. [DOI] [PubMed] [Google Scholar]














