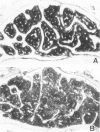
Abstract
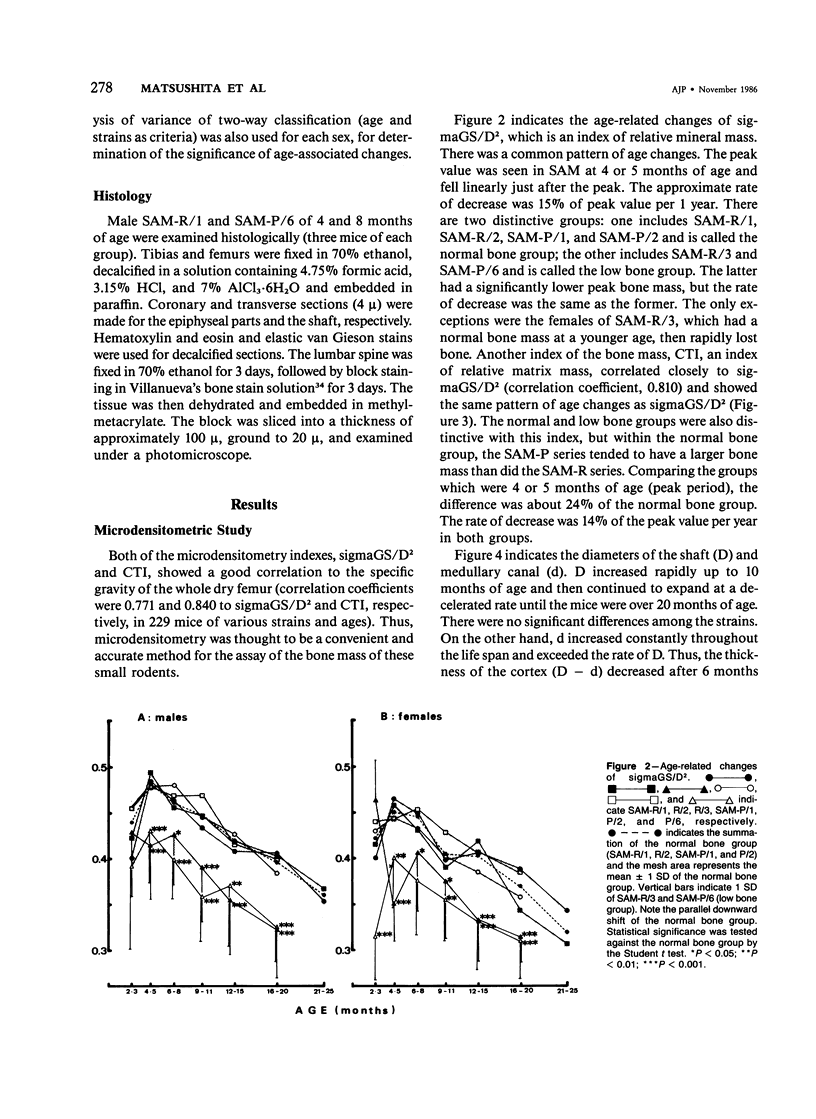
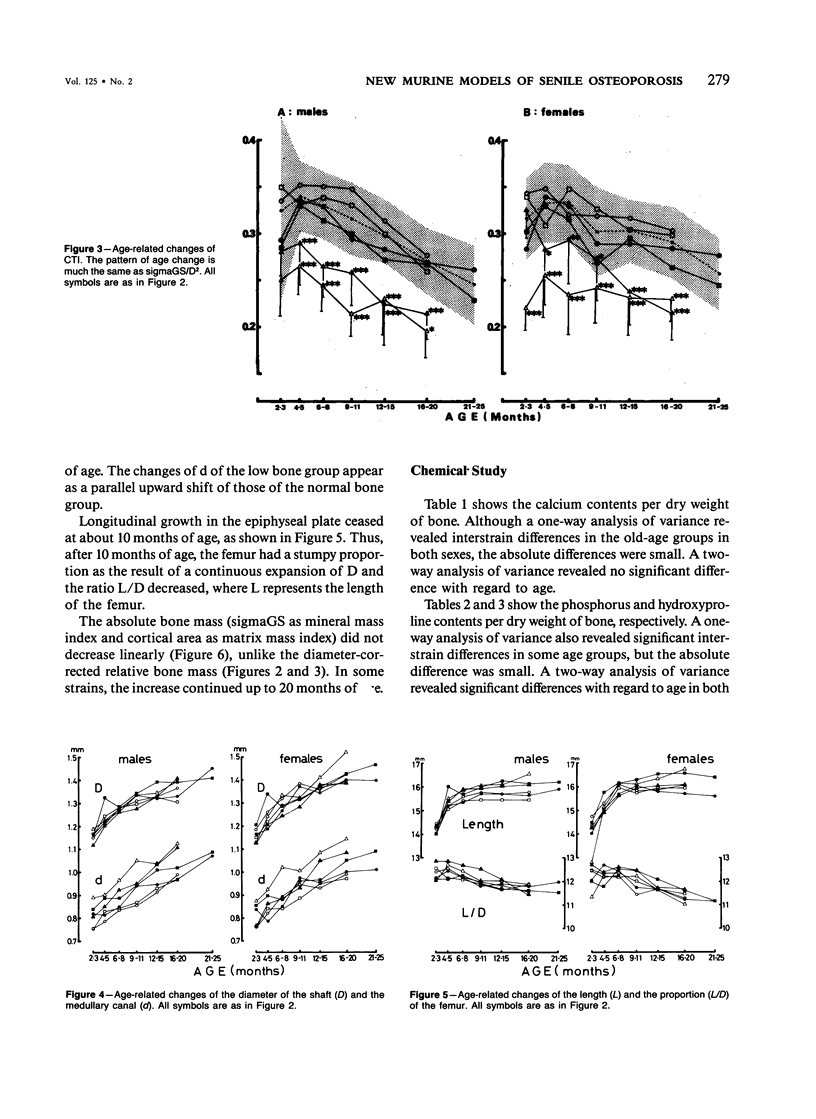
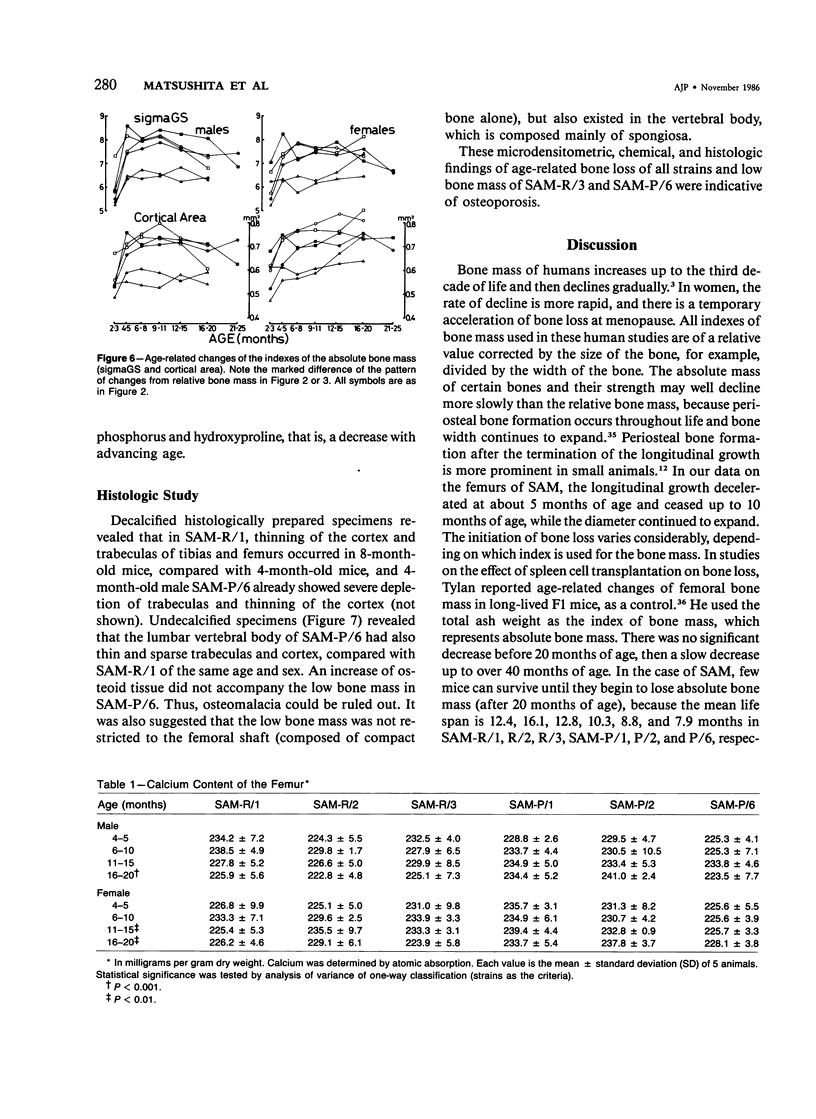
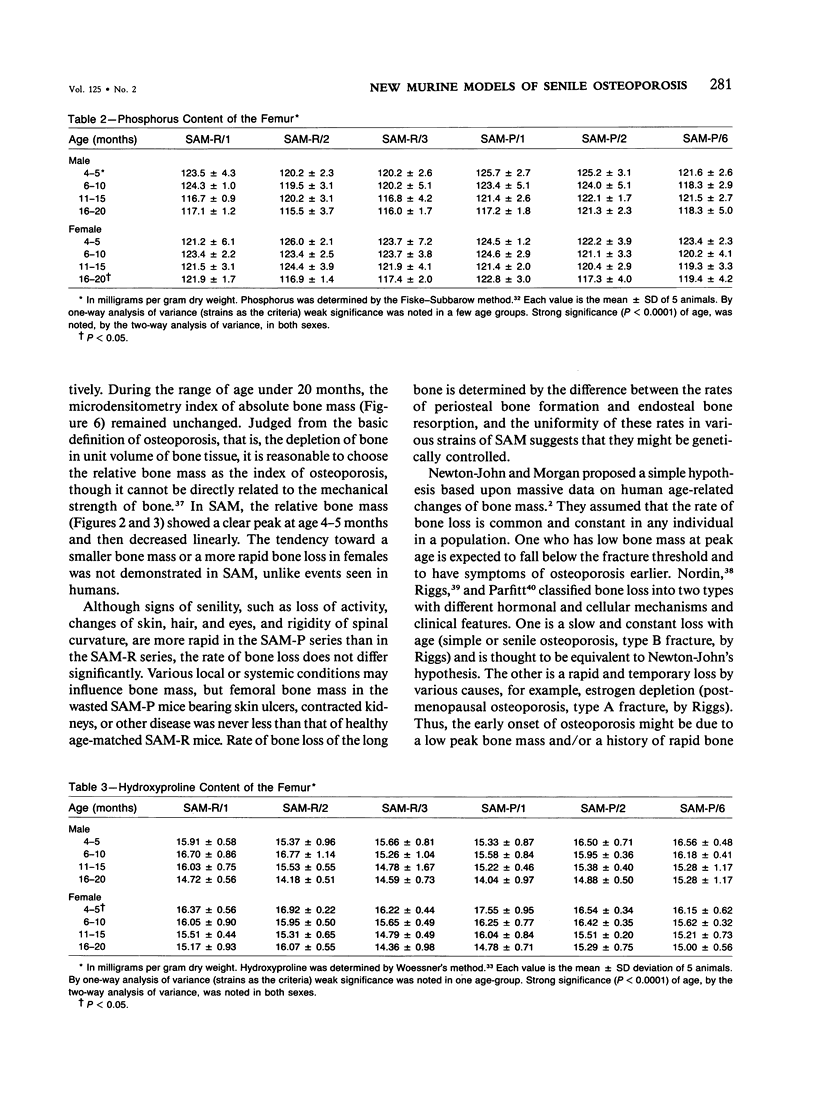
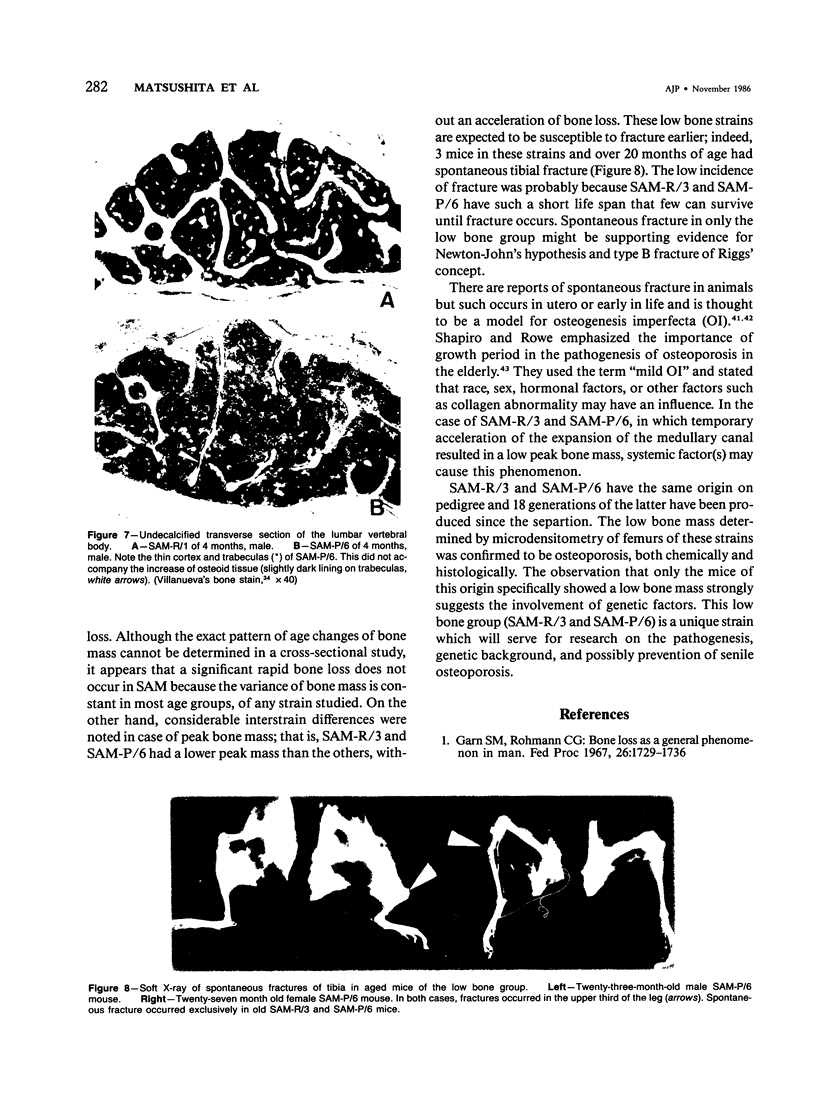
Age-related changes of the femoral bone mass in several strains of the senescence-accelerated mouse (SAM) were investigated. Microdensitometrically, all strains exhibited essentially the same patterns of age changes, that is, bone mass corrected by the diameter of the shaft reached the peak value when the mice were 4 or 5 months of age and then fell linearly with age up to over 20 months of age. Two strains, SAM-R/3 and SAM-P/6, which originated from the same ancestry on pedigree, had a significantly lower peak bone mass than other strains (SAM-R/1, SAM-R/2, SAM-P/1, and SAM-P/2). On the other hand, the strains with a low peak bone mass had the same rate of decrease as other strains. Mineral and collagen contents per dry weight of bone showed little difference among the strains. Histologic studies of tibia, femur, and lumbar spine revealed that the osteopenia was not due to osteomalacia but, rather, to osteoporosis. The elderly mice in these two strains were prone to fracture, thus should be important models for study of senile osteoporosis seen clinically.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garn S. M., Rohmann C. G., Wagner B. Bone loss as a general phenomenon in man. Fed Proc. 1967 Nov-Dec;26(6):1729–1736. [PubMed] [Google Scholar]
- Guenet J. L., Stanescu R., Maroteaux P., Stanescu V. Fragilitas ossium: a new autosomal recessive mutation in the mouse. J Hered. 1981 Nov-Dec;72(6):440–441. doi: 10.1093/oxfordjournals.jhered.a109554. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Hashimoto K., Honma A., Takeshita S., Hosokawa M., Yasuhira K., Takeda T. Isolation and characterization of senile amyloid--related antigenic substance (SASSAM) from mouse serum. Apo SASSAM is a low molecular weight apoprotein of high density lipoprotein. J Exp Med. 1983 Nov 1;158(5):1600–1614. doi: 10.1084/jem.158.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Honma A., Takeshita S., Hashimoto K., Hosokawa M., Yasuhira K., Takeda T. Systemic senile amyloid in senescence-accelerated mice. A unique fibril protein demonstrated in tissues from various organs by the unlabeled immunoperoxidase method. Lab Invest. 1983 Feb;48(2):231–240. [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Honma A., Toda K., Takeshita S., Matsushita M., Yonezu T., Hosokawa M., Takeda T. Age-related changes of serum apoprotein SASSAM, apoprotein A-I and low-density lipoprotein levels in senescence accelerated mouse (SAM). Mech Ageing Dev. 1984 Aug;26(2-3):311–326. doi: 10.1016/0047-6374(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Hooper A. C. Skeletal dimensions in senescent laboratory mice. Gerontology. 1983;29(4):221–225. doi: 10.1159/000213120. [DOI] [PubMed] [Google Scholar]
- Hosokawa M., Kasai R., Higuchi K., Takeshita S., Shimizu K., Hamamoto H., Honma A., Irino M., Toda K., Matsumura A. Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech Ageing Dev. 1984 Jul;26(1):91–102. doi: 10.1016/0047-6374(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Hosokawa M., Takeshita S., Higuchi K., Shimizu K., Irino M., Toda K., Honma A., Matsumura A., Yasuhira K., Takeda T. Cataract and other ophthalmic lesions in senescence accelerated mouse (SAM). Morphology and incidence of senescence associated ophthalmic changes in mice. Exp Eye Res. 1984 Feb;38(2):105–114. doi: 10.1016/0014-4835(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kusida K., Miyamoto S., Sumi Y., Orimo H., Yamashita G. Quantitative assessment of bone density on X-ray picture. Nihon Seikeigeka Gakkai Zasshi. 1983 Dec;57(12):1923–1936. [PubMed] [Google Scholar]
- Kalu D. N. Evaluation of the pathogenesis of skeletal changes in ovariectomized rats. Endocrinology. 1984 Aug;115(2):507–512. doi: 10.1210/endo-115-2-507. [DOI] [PubMed] [Google Scholar]
- MEEMA H. E., HARRIS C. K., PORRETT R. E. A METHOD FOR DETERMINATION OF BONE-SALT CONTENT OF CORTICAL BONE. Radiology. 1964 Jun;82:986–997. doi: 10.1148/82.6.986. [DOI] [PubMed] [Google Scholar]
- Matsumura A., Higuchi K., Shimizu K., Hosokawa M., Hashimoto K., Yasuhira K., Takeda T. A novel amyloid fibril protein isolated from senescence-accelerated mice. Lab Invest. 1982 Sep;47(3):270–275. [PubMed] [Google Scholar]
- Mazess R. B. On aging bone loss. Clin Orthop Relat Res. 1982 May;(165):239–252. [PubMed] [Google Scholar]
- Newton-John H. F., Morgan D. B. The loss of bone with age, osteoporosis, and fractures. Clin Orthop Relat Res. 1970;71:229–252. [PubMed] [Google Scholar]
- Nordin B. E. Clinical significance and pathogenesis of osteoporosis. Br Med J. 1971 Mar 13;1(5749):571–576. doi: 10.1136/bmj.1.5749.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin B. E. International patterns of osteoporosis. Clin Orthop Relat Res. 1966 Mar-Apr;45:17–30. [PubMed] [Google Scholar]
- Parfitt A. M. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int. 1984;36 (Suppl 1):S123–S128. doi: 10.1007/BF02406145. [DOI] [PubMed] [Google Scholar]
- Rao G. V., Draper H. H. Age-related changes in the bones of adult mice. J Gerontol. 1969 Apr;24(2):149–151. doi: 10.1093/geronj/24.2.149. [DOI] [PubMed] [Google Scholar]
- Riesenfeld A. Age changes on bone size and mass in two strains of senescent rats. Acta Anat (Basel) 1981;109(1):64–69. doi: 10.1159/000145366. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd Evidence for two distinct syndromes of involutional osteoporosis. Am J Med. 1983 Dec;75(6):899–901. doi: 10.1016/0002-9343(83)90860-4. [DOI] [PubMed] [Google Scholar]
- Ruff C. B., Hayes W. C. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science. 1982 Sep 3;217(4563):945–948. doi: 10.1126/science.7112107. [DOI] [PubMed] [Google Scholar]
- SILBERBERG M., SILBERBERG R. Osteoarthrosis and osteoporosis in senile mice. Gerontologia. 1962;6:91–101. doi: 10.1159/000211110. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF L. Joint diseases of laboratory animals. J Natl Cancer Inst. 1958 May;20(5):965–977. [PubMed] [Google Scholar]
- Saville P. D. Changes in skeletal mass and fragility with castration in the rat; a model of osteoporosis. J Am Geriatr Soc. 1969 Feb;17(2):155–166. doi: 10.1111/j.1532-5415.1969.tb03169.x. [DOI] [PubMed] [Google Scholar]
- Saville P. D., Smith R. Bone density, breaking force and leg muscle mass as functions of weight in bipedal rats. Am J Phys Anthropol. 1966 Jul;25(1):35–39. doi: 10.1002/ajpa.1330250105. [DOI] [PubMed] [Google Scholar]
- Shah B. G., Krishnarao G. V., Draper H. H. The relationship of Ca and P nutrition during adult life and osteoporosis in aged mice. J Nutr. 1967 May;92(1):30–42. doi: 10.1093/jn/92.1.30. [DOI] [PubMed] [Google Scholar]
- Shapiro J. R., Rowe D. W. Imperfect osteogenesis and osteoporosis. N Engl J Med. 1984 Jun 28;310(26):1738–1740. doi: 10.1056/NEJM198406283102610. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Ishii M., Yamamuro T., Takeshita S., Hosokawa M., Takeda T. Amyloid deposition in intervertebral discs of senescence-accelerated mouse. Arthritis Rheum. 1982 Jun;25(6):710–712. doi: 10.1002/art.1780250618. [DOI] [PubMed] [Google Scholar]
- Simon M. R. The rat as an animal model for the study of senile idiopathic osteoporosis. Acta Anat (Basel) 1984;119(4):248–250. doi: 10.1159/000145893. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Nance W. E., Kang K. W., Christian J. C., Johnston C. C., Jr Genetic factors in determining bone mass. J Clin Invest. 1973 Nov;52(11):2800–2808. doi: 10.1172/JCI107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. W., Jr, Rizek J. Epidemiologic studies of osteoporosis in Women of Puerto Rico and southeastern Michigan with special reference to age, race, national origin and to other related or associated findings. Clin Orthop Relat Res. 1966 Mar-Apr;45:31–48. [PubMed] [Google Scholar]
- Solomon L. Bone density in ageing Caucasian and African populations. Lancet. 1979 Dec 22;2(8156-8157):1326–1330. doi: 10.1016/s0140-6736(79)92813-7. [DOI] [PubMed] [Google Scholar]
- Takeda T., Hosokawa M., Takeshita S., Irino M., Higuchi K., Matsushita T., Tomita Y., Yasuhira K., Hamamoto H., Shimizu K. A new murine model of accelerated senescence. Mech Ageing Dev. 1981 Oct;17(2):183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Higuchi K., Hosokawa M., Matsumura A., Higuchi K., Kohno A., Matsushita M., Yonezu T., Takeda T. Morphologic demonstration of cytoplasmic ASSAM-related antigenic substance (CASSAM) by an immunoperoxidase technique. Am J Pathol. 1985 Dec;121(3):455–465. [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Hosokawa M., Irino M., Higuchi K., Shimizu K., Yasuhira K., Takeda T. Spontaneous age-associated amyloidosis in senescence-accelerated mouse (SAM). Mech Ageing Dev. 1982 Sep;20(1):13–23. doi: 10.1016/0047-6374(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Tyan M. L. Age-related osteopenia: evidence for an intrinsic defect of bone resorbing cells and a possible treatment. Proc Soc Exp Biol Med. 1985 Jun;179(2):240–247. doi: 10.3181/00379727-179-42093. [DOI] [PubMed] [Google Scholar]
- Villanueva A. R. A bone stain for osteoid seams in fresh, unembedded, mineralized bone. Stain Technol. 1974 Jan;49(1):1–8. doi: 10.3109/10520297409116928. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Underwood J. L., Hutson M. S., DeLuca H. F. Bone histomorphometry in vitamin D-deficient rats infused with calcium and phosphorus. Am J Physiol. 1984 Jun;246(6 Pt 1):E499–E505. doi: 10.1152/ajpendo.1984.246.6.E499. [DOI] [PubMed] [Google Scholar]