Abstract
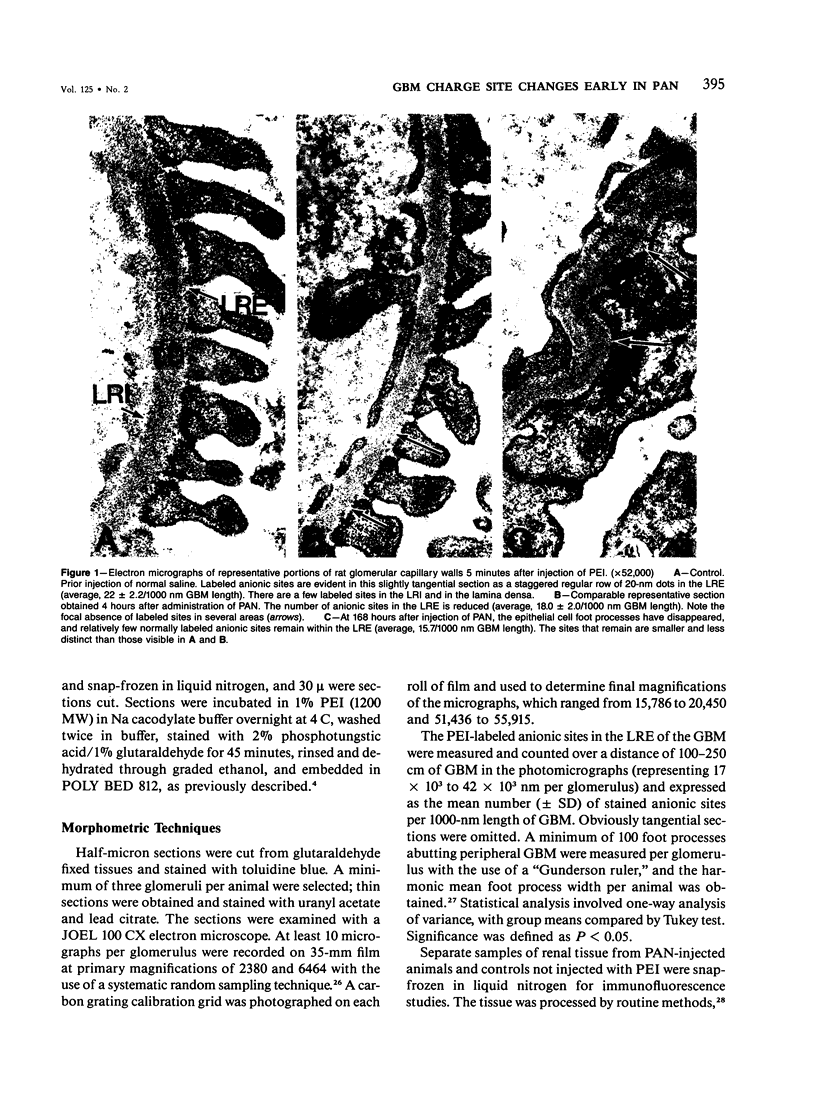
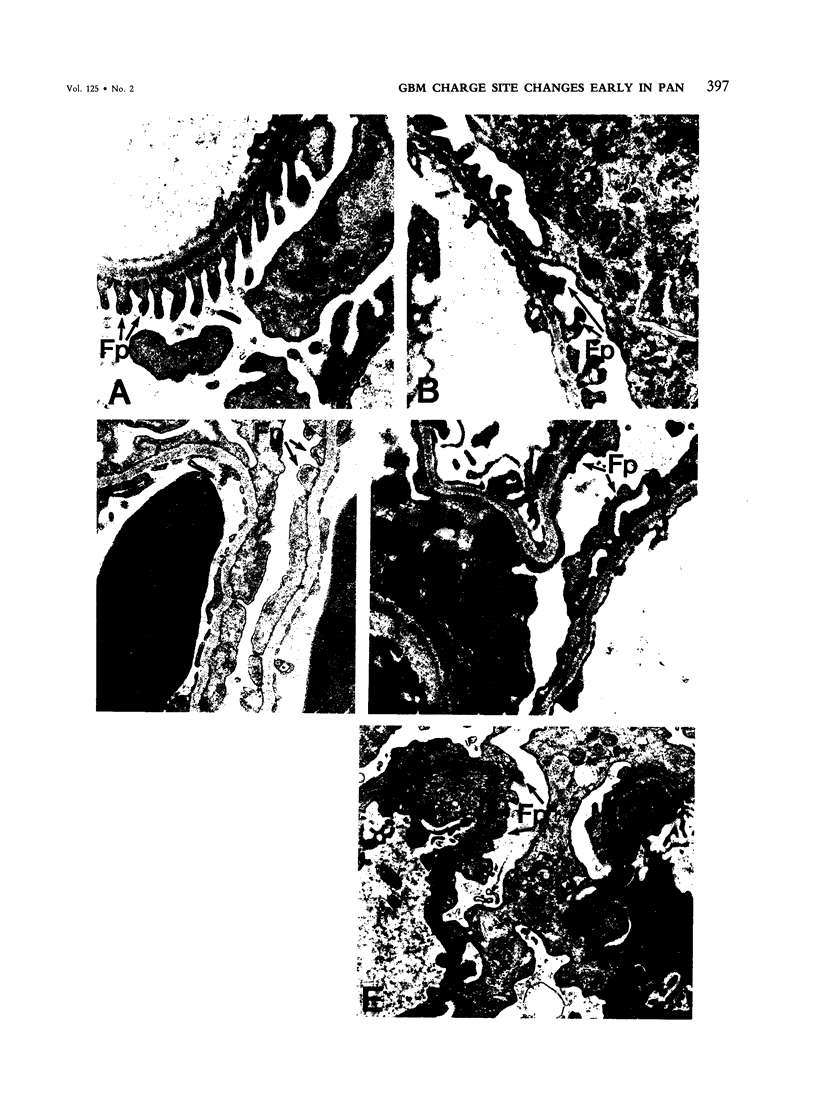
Alterations of glomerular basement membrane (GBM) anionic (charge sites, CSs) in the development of proteinuria in a model of idiopathic nephrotic syndrome in man (puromycin aminonucleoside nephrotic syndrome [PAN] in the rat) were assessed quantitatively and sequentially early after disease induction. GBM CSs (known to consist mainly of heparan sulfate-rich proteoglycans) were stained in vivo and, in a separate group of animals by an in vitro method, with the cationic marker polyethyleneimine (PEI) studied by electron microscopic examination. Four hours after administration of PAN, there was a significant decrease in GBM lamina rara externa CSs: 18 +/- 0.7 versus 22.0 +/- 2.2 per 1000 nm GBM in controls by PEI injection and 17.2 +/- 2.7 versus 21.1 +/- 1.6 per 1000 nm GBM in controls by PEI in vitro staining. This CS alteration coincided with changes in glomerular epithelial cell morphologic characteristics (increased cytoplasmic organelles and rough endoplasmic reticulum) and preceded the detection of foot process broadening (at 24 hours) and increased urinary albuminuria (suggested at 12-24 hours, statistically significant at 36-48 hours). These results suggest that GBM CS-heparan sulfate proteoglycan alterations consisting of either decreased number and/or less anionic charge occur early in PAN and support a role for glomerular epithelial cell maintenance of GBM CS for normal glomerular function.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avasthi P. S., Evan A. P. Glomerular permeability in aminonucleoside-induced nephrosis in rats. A proposed role of endothelial cells. J Lab Clin Med. 1979 Feb;93(2):266–276. [PubMed] [Google Scholar]
- Bertolatus J. A., Foster S. J., Hunsicker L. G. Stainable glomerular basement membrane polyanions and renal hemodynamics during hexadimethrine-induced proteinuria. J Lab Clin Med. 1984 Apr;103(4):632–642. [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Charonis A. S., Tsilibary P. C., Kramer R. H., Wissig S. L. Localization of heparan sulfate proteoglycan in the basement membrane of continuous capillaries. Microvasc Res. 1983 Jul;26(1):108–115. doi: 10.1016/0026-2862(83)90059-6. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Karnovsky M. J. Effects of the aminonucleoside of puromycin on glomerular epithelial cells in vitro. Am J Pathol. 1985 Mar;118(3):398–407. [PMC free article] [PubMed] [Google Scholar]
- Gang N. F., Trachtenberg E., Wheatley P. J., Mautner W. Glomerular basement membrane damage in aminonucleoside nephrosis as visualized by lanthanum. Proc Soc Exp Biol Med. 1972 Jun;140(2):449–453. doi: 10.3181/00379727-140-36477. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Seefeldt T., Osterby R. Glomerular epithelial foot processes in normal man and rats. Distribution of true width and its intra- and inter-individual variation. Cell Tissue Res. 1980;205(1):147–155. doi: 10.1007/BF00234450. [DOI] [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Shaffer S. J. Acute reversible proteinuria induced by infusion of the polycation hexadimethrine. Kidney Int. 1981 Jul;20(1):7–17. doi: 10.1038/ki.1981.98. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S. Biophysiology of glomerular filtration and proteinuria. Lab Invest. 1984 Jul;51(1):7–21. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Jakubowski M. L. Unaltered anionic sites of glomerular basement membrane in aminonucleoside nephrosis. Kidney Int. 1984 Apr;25(4):613–618. doi: 10.1038/ki.1984.65. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. J., Dehnel P. J., Oegema T. R., Brown D. M. Alterations in proteoglycan metabolism in the nephrotic syndrome induced by the aminonucleoside of puromycin. Lab Invest. 1984 May;50(5):543–551. [PubMed] [Google Scholar]
- Linker A., Hovingh P., Kanwar Y. S., Farquhar M. G. Characterization of heparan sulfate isolated from drug glomerular basement membranes. Lab Invest. 1981 Jun;44(6):560–565. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Fish A. J., Day N. K., Michael A. F. The glomerular mesangium. II. Studies of macromolecular uptake in nephrotoxic nephritis in rats. J Clin Invest. 1974 Feb;53(2):431–439. doi: 10.1172/JCI107577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick G. F., Ladoulis C. T., Cavallo T. Decreased anionic groups and increased permeability precedes deposition of immune complexes in the glomerular capillary wall. Am J Pathol. 1981 Nov;105(2):114–120. [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mynderse L. A., Hassell J. R., Kleinman H. K., Martin G. R., Martinez-Hernandez A. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest. 1983 Mar;48(3):292–302. [PubMed] [Google Scholar]
- Nevins T. E., Gaston T., Basgen J. M. Quantitative indexes of aminonucleoside-induced nephrotic syndrome. Am J Pathol. 1984 Oct;117(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Mende C. W. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int. 1972;1(1):3–11. doi: 10.1038/ki.1972.2. [DOI] [PubMed] [Google Scholar]
- Olson J. L., Rennke H. G., Venkatachalam M. A. Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab Invest. 1981 Mar;44(3):271–279. [PubMed] [Google Scholar]
- Pilia P. A., Boackle R. J., Swain R. P., Ainsworth S. K. Complement-independent nephrotoxic serum nephritis in Munich Wistar rats. Immunologic and ultrastructural studies. Lab Invest. 1983 May;48(5):585–597. [PubMed] [Google Scholar]
- Pilia P. A., Swain R. P., Williams A. V., Loadholt C. B., Ainsworth S. K. Glomerular anionic site distribution in nonproteinuric rats. A computer-assisted morphometric analysis. Am J Pathol. 1985 Dec;121(3):474–485. [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Schurer J. W., Hoedemaeker J., Molenaar I. Polyethyleneimine as tracer particle for (immuno) electron microscopy. J Histochem Cytochem. 1977 May;25(5):384–387. doi: 10.1177/25.5.325123. [DOI] [PubMed] [Google Scholar]
- Schwartz M. M., Sharon Z., Pauli B. U., Lewis E. J. Inhibition of glomerular visceral epithelial cell endocytosis during nephrosis induced by puromycin aminonucleoside. Lab Invest. 1984 Dec;51(6):690–696. [PubMed] [Google Scholar]
- Seiler M. W., Hoyer J. R., Krueger T. E. Altered localization of protamine-heparin complexes in aminonucleoside nephrosis. Lab Invest. 1980 Jul;43(1):9–17. [PubMed] [Google Scholar]
- Suzuki Y., Maruyama Y., Arakawa M., Oite T. Preservation of fixed anionic sites in the GBM in the acute proteinuric phase of cationic antigen mediated in-situ immune complex glomerulonephritis in the rat. Histochemistry. 1984;81(3):243–246. doi: 10.1007/BF00495634. [DOI] [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velosa J. A., Glasser R. J., Nevins T. E., Michael A. F. Experimental model of focal sclerosis. II. Correlation with immunopathologic changes, macromolecular kinetics, and polyanion loss. Lab Invest. 1977 May;36(5):527–534. [PubMed] [Google Scholar]
- Vernier R. L., Klein D. J., Sisson S. P., Mahan J. D., Oegema T. R., Brown D. M. Heparan sulfate--rich anionic sites in the human glomerular basement membrane. Decreased concentration in congenital nephrotic syndrome. N Engl J Med. 1983 Oct 27;309(17):1001–1009. doi: 10.1056/NEJM198310273091701. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol. 1975 Dec;67(3):660–674. doi: 10.1083/jcb.67.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]