Abstract
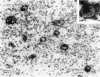
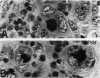
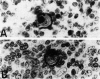
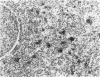
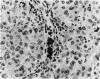
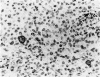
The immunoreactivity of six different monoclonal antigranulocyte antibodies (Leu M1, TG1, 3C4, BY/87a, BY/37a, and 3CD1) has been evaluated in 23 cases of Hodgkin's disease (7 lymphocyte predominant, 12 nodular sclerosing, and 5 mixed cellularity); in a variety of non-Hodgkin's lymphomas and in a series of reactive and benign lesions of lymph nodes. Applying a monoclonal antibody (PD7/26) to leukocyte common antigen (T200), we have also investigated reports that the L&H variants in nodular lymphocyte predominant Hodgkin's disease are strongly immunoreactive for leukocyte common antigen in contrast to the lack of reactivity of Reed-Sternberg (RS) cells and variants thereof in other forms of Hodgkin's disease. All six monoclonal anti-granulocyte antibodies reacted against RS cells and "Hodgkin's cells" in the nodular sclerosing (NSHD) and mixed cellularity (MCHD) types, with strong cell membrane and juxtanuclear (Golgi) staining. In contrast an anti-leukocyte antibody PD7/26 was unreactive with RS cells and variants thereof in NSHD and MCHD. On the other hand, RS cells and L&H variants thereof in the nodular L&H form of Hodgkin's disease (nodular lymphocyte predominant type) showed reactivity with PD7/26 but not with the anti-granulocyte markers. Rare L&H cells in 2 cases of diffuse lymphocyte predominant type showed reactivity with some, but not all, of the anti-granulocyte antibodies. These findings provide further support for the concept that the nodular L&H type of Hodgkin's disease represents an entity distinct from other forms of this disorder. Our studies also demonstrate the usefulness of these immunoperoxidase techniques when applied to formalinfixed, paraffin-embedded tissues.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulaziz Z., Mason D. Y., Stein H., Gatter K. C., Nash J. R. An immunohistological study of the cellular constituents of Hodgkin's disease using a monoclonal antibody panel. Histopathology. 1984 Jan;8(1):1–25. doi: 10.1111/j.1365-2559.1984.tb02318.x. [DOI] [PubMed] [Google Scholar]
- Battifora H., Trowbridge I. S. A monoclonal antibody useful for the differential diagnosis between malignant lymphoma and nonhematopoietic neoplasms. Cancer. 1983 Mar 1;51(5):816–821. doi: 10.1002/1097-0142(19830301)51:5<816::aid-cncr2820510512>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Beckstead J. H., Warnke R., Bainton D. F. Histochemistry of Hodgkin's disease. Cancer Treat Rep. 1982 Apr;66(4):609–613. [PubMed] [Google Scholar]
- Burns B. F., Colby T. V., Dorfman R. F. Differential diagnostic features of nodular L & H Hodgkin's disease, including progressive transformation of germinal centers. Am J Surg Pathol. 1984 Apr;8(4):253–261. doi: 10.1097/00000478-198404000-00002. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- DORFMAN R. F. Enzyme histochemistry of the cells in Hodgkin's disease and allied disorders. Nature. 1961 Jun 3;190:925–926. doi: 10.1038/190925a0. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Doggett R. S., Colby T. V., Dorfman R. F. Interfollicular Hodgkin's disease. Am J Surg Pathol. 1983 Mar;7(2):145–149. doi: 10.1097/00000478-198303000-00004. [DOI] [PubMed] [Google Scholar]
- Dorfman R. F., Rice D. F., Mitchell A. D., Kempson R. L., Levine G. Ultrastructural studies of Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:221–238. [PubMed] [Google Scholar]
- Dorfman R. F., Warnke R. Lymphadenopathy simulating the malignant lymphomas. Hum Pathol. 1974 Sep;5(5):519–550. doi: 10.1016/s0046-8177(74)80005-5. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Alcock C., Heryet A., Mason D. Y. Clinical importance of analysing malignant tumours of uncertain origin with immunohistological techniques. Lancet. 1985 Jun 8;1(8441):1302–1305. doi: 10.1016/s0140-6736(85)92794-1. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Isaacson P. Immunochemical demonstration of J chain: a marker of B-cell malignancy. J Clin Pathol. 1979 Aug;32(8):802–807. doi: 10.1136/jcp.32.8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E. Common activated helper-T-cell origin for lymphomatoid papulosis, mycosis fungoides, and some types of Hodgkin's disease. Lancet. 1985 Oct 19;2(8460):864–865. doi: 10.1016/s0140-6736(85)90128-x. [DOI] [PubMed] [Google Scholar]
- Kadin M. E. Possible origin of the Reed-Sternberg cell from an interdigitating reticulum cell. Cancer Treat Rep. 1982 Apr;66(4):601–608. [PubMed] [Google Scholar]
- Kurtin P. J., Pinkus G. S. Leukocyte common antigen--a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Hum Pathol. 1985 Apr;16(4):353–365. doi: 10.1016/s0046-8177(85)80229-x. [DOI] [PubMed] [Google Scholar]
- Lukes R. J., Butler J. J. The pathology and nomenclature of Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1063–1083. [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985 Jan;118(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Thomas P., Said J. W. Leu-M1--a marker for Reed-Sternberg cells in Hodgkin's disease. An immunoperoxidase study of paraffin-embedded tissues. Am J Pathol. 1985 May;119(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Kaiserling E., Lennert K. Nodular paragranuloma and progressively transformed germinal centers. Ultrastructural and immunohistologic findings. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;31(3):211–225. doi: 10.1007/BF02889938. [DOI] [PubMed] [Google Scholar]
- Poppema S. The diversity of the immunohistological staining pattern of Sternberg-Reed cells. J Histochem Cytochem. 1980 Aug;28(8):788–791. doi: 10.1177/28.8.6777426. [DOI] [PubMed] [Google Scholar]
- Rappaport H., Berard C. W., Butler J. J., Dorfman R. F., Lukes R. J., Thomas L. B. Report of the Committee on Histopathological Criteria Contributing to Staging of Hodgkin's Disease. Cancer Res. 1971 Nov;31(11):1864–1865. [PubMed] [Google Scholar]
- Schienle H. W., Stein N., Müller-Ruchholtz W. Neutrophil granulocytic cell antigen defined by a monoclonal antibody--its distribution within normal haemic and non-haemic tissue. J Clin Pathol. 1982 Sep;35(9):959–966. doi: 10.1136/jcp.35.9.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Lemke H., Mason D. Y. Evidence of Sternberg-Reed cells being derived from activated lymphocytes. Haematol Blood Transfus. 1985;29:441–444. doi: 10.1007/978-3-642-70385-0_91. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Stein H., Uchánska-Ziegler B., Gerdes J., Ziegler A., Wernet P. Hodgkin and Sternberg-Reed cells contain antigens specific to late cells of granulopoiesis. Int J Cancer. 1982 Mar 15;29(3):283–290. doi: 10.1002/ijc.2910290310. [DOI] [PubMed] [Google Scholar]
- Warnke R. A., Gatter K. C., Falini B., Hildreth P., Woolston R. E., Pulford K., Cordell J. L., Cohen B., De Wolf-Peeters C., Mason D. Y. Diagnosis of human lymphoma with monoclonal antileukocyte antibodies. N Engl J Med. 1983 Nov 24;309(21):1275–1281. doi: 10.1056/NEJM198311243092102. [DOI] [PubMed] [Google Scholar]