Abstract
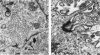
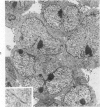
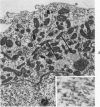
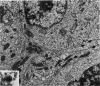
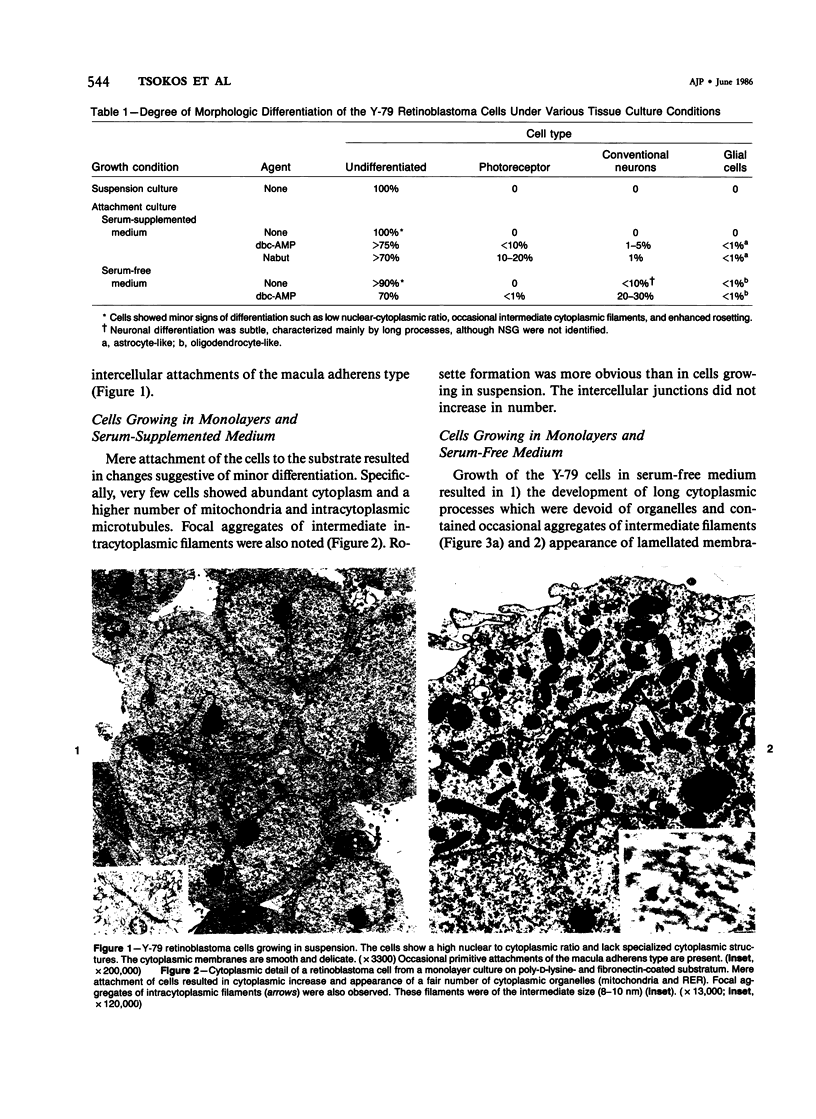
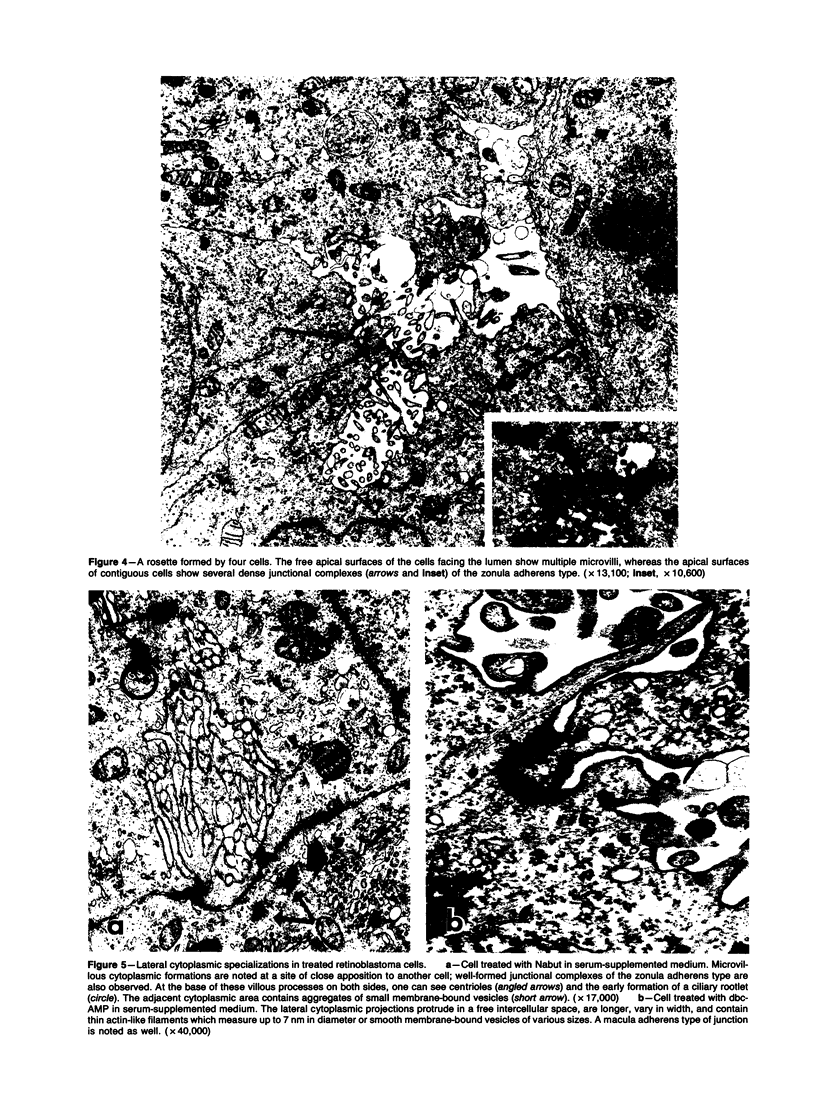
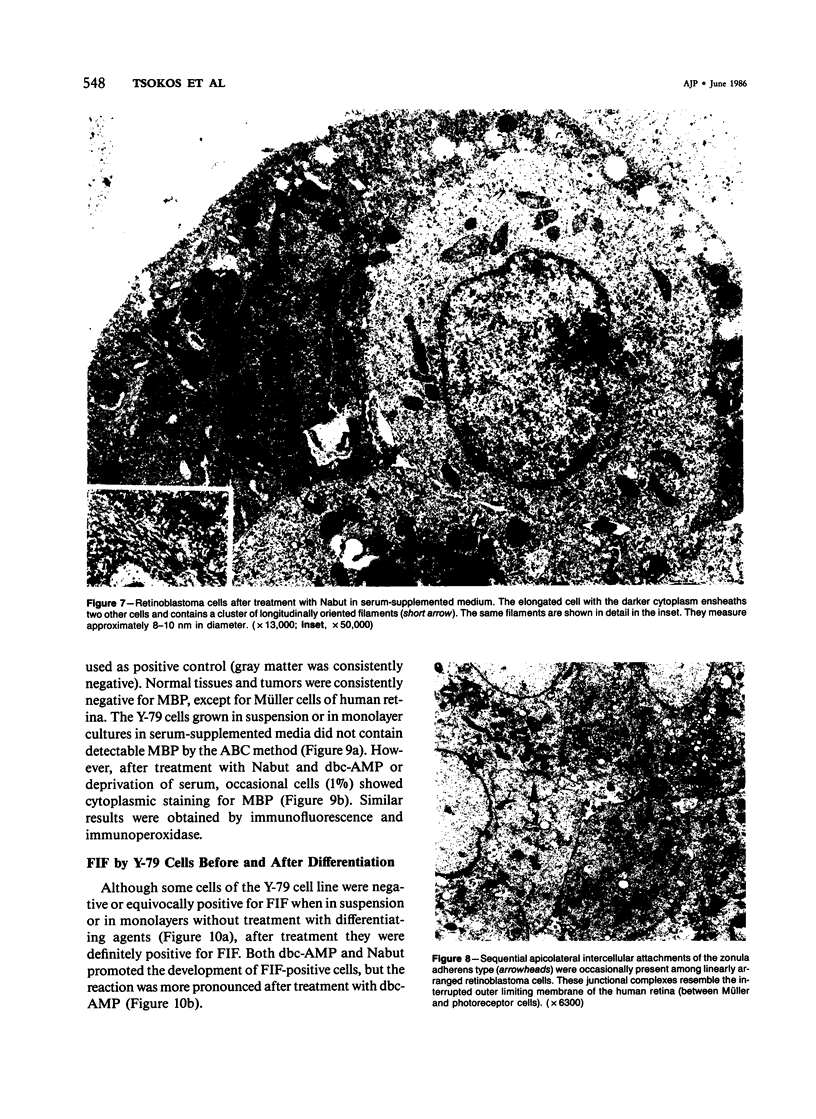
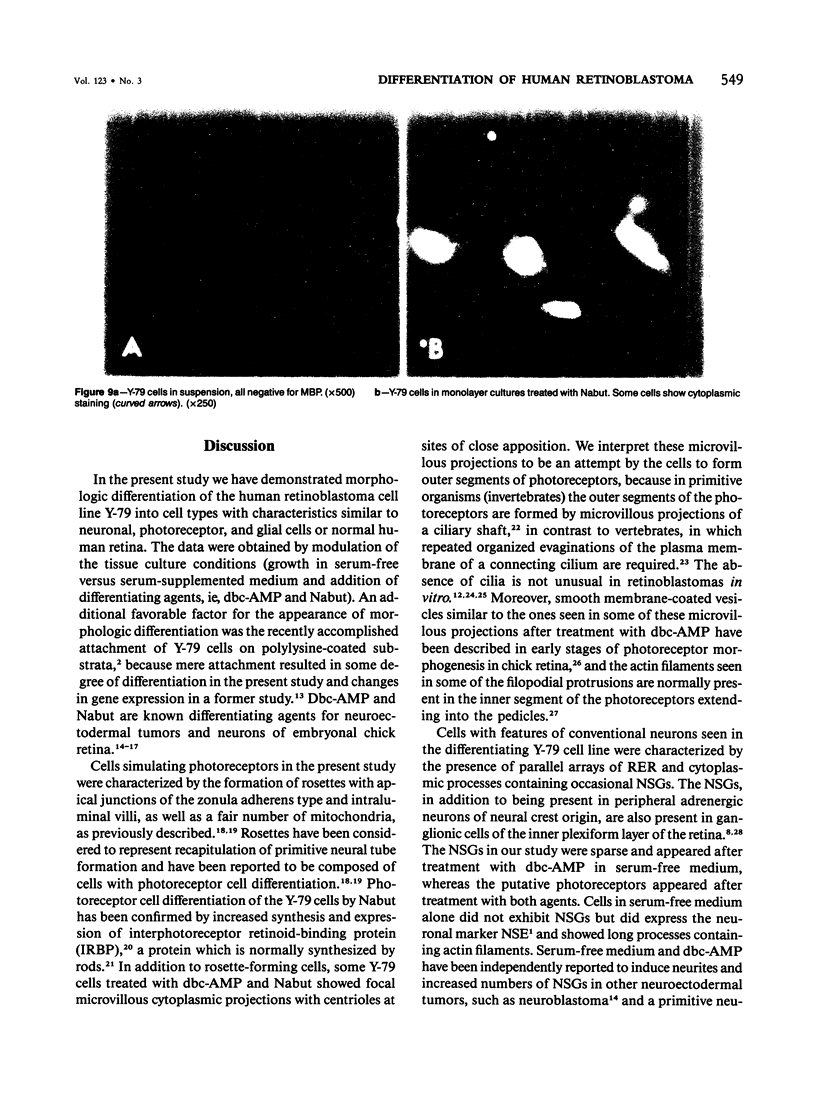
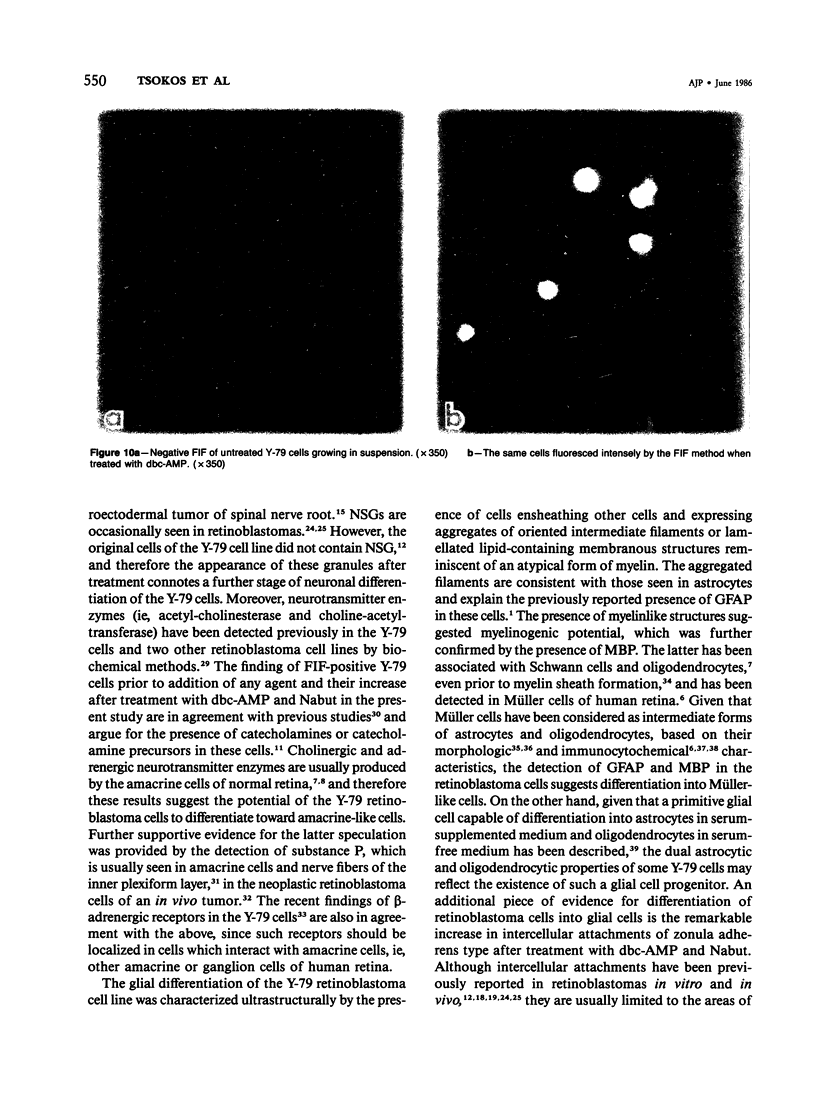
The capacity of a primitive human retinoblastoma cell line (Y-79) to differentiate into several cell types of normal human retina was investigated. Cells were studied in suspension and monolayer cultures, in serum-free or serum-supplemented medium, and in the presence or absence of differentiating agents such as N6O12-dibutyryl adenosine 3',5'-cyclic monophosphate (dbc-AMP) and sodium butyrate (Nabut). Electron microscopy, immunohistochemistry for detection of myelin basic protein (MBP), and formaldehyde-induced fluorescence (FIF) for catecholamines were performed. Treated cells exhibited morphologic characteristics supportive of differentiation toward photoreceptors, conventional neurons and glial cells, increased FIF reactivity, and MBP expression. Growth in serum-free medium without differentiating agents led to a similar but less enhanced morphologic differentiation. These results confirm the concept that human retinoblastoma originates from a primitive neuroectodermal multipotential cell.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemer F. A., Vlug A. M., Rijksen G., Hamburg A., Staal G. E. Characterization of some glycolytic enzymes from human retina and retinoblastoma. Cancer Res. 1982 Oct;42(10):4228–4232. [PubMed] [Google Scholar]
- Bignami A., Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979 Jan;28(1):63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- Brecha N., Hendrickson A., Florén I., Karten H. J. Localization of substance P-like immunoreactivity within the monkey retina. Invest Ophthalmol Vis Sci. 1982 Aug;23(2):147–153. [PubMed] [Google Scholar]
- Char D. H., Wood I. S., Huhta K., Rand N., Morita C. T., Howes E. L., Jr Retinoblastoma: tissue culture lines and monoclonal antibody studies. Invest Ophthalmol Vis Sci. 1984 Jan;25(1):30–40. [PubMed] [Google Scholar]
- De Vitry F., Picart R., Jacque C., Legault L., Dupouey P., Tixier-Vidal A. Presumptive common precursor for neuronal and glial cell lineages in mouse hypothalamus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4165–4169. doi: 10.1073/pnas.77.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINE B. S., ZIMMERMAN L. E. Muller's cells and the "middle limiting membrane" of the human retina. An electron microscopic study. Invest Ophthalmol. 1962 Jun;1:304–326. [PubMed] [Google Scholar]
- Greiner J. V., Weidman T. A. Embryogenesis of the rabbit retina. Exp Eye Res. 1982 May;34(5):749–765. doi: 10.1016/s0014-4835(82)80035-3. [DOI] [PubMed] [Google Scholar]
- Grün G. The ultrastructural differentiation of synaptic sites in the inner plexiform layer of a teleostean retina. Z Mikrosk Anat Forsch. 1977;91(4):687–703. [PubMed] [Google Scholar]
- Hollyfield J. G., Fliesler S. J., Rayborn M. E., Fong S. L., Landers R. A., Bridges C. D. Synthesis and secretion of interstitial retinol-binding protein by the human retina. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):58–67. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Sugihara Y., Nishimura Y., Shimai K. Atypical neural sheaths formed by Müller cells in chicken retina. Okajimas Folia Anat Jpn. 1980 Aug;57(2-3):79–88. doi: 10.2535/ofaj1936.57.2-3_79. [DOI] [PubMed] [Google Scholar]
- Ishikawa S., Ohshima Y., Suzuki T., Oboshi S. Primitive neuroectodermal tumor (neuroepithelioma) of spinal nerve root -- Report of an adult case and establishment of a cell line. Acta Pathol Jpn. 1979 Mar;29(2):289–301. doi: 10.1111/j.1440-1827.1979.tb03183.x. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Lim R., Blodi F. C. Dual properties of cultured retinoblastoma cells: immunohistochemical characterization of neuronal and glial markers. Exp Eye Res. 1984 Aug;39(2):207–215. doi: 10.1016/0014-4835(84)90009-5. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Triche T. J., Chader G. J. Retinoblastoma--origin from a primitive neuroectodermal cell? Nature. 1984 Feb 2;307(5950):471–473. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Wiggert B., Lee L., Chader G. J. Butyrate enhances the synthesis of interphotoreceptor retinoid-binding protein (IRBP) by Y-79 human retinoblastoma cells. J Cell Physiol. 1985 Aug;124(2):233–239. doi: 10.1002/jcp.1041240210. [DOI] [PubMed] [Google Scholar]
- Kyritsis A., Joseph G., Chader G. J. Effects of butyrate, retinol, and retinoic acid on human Y-79 retinoblastoma cells growing in monolayer cultures. J Natl Cancer Inst. 1984 Sep;73(3):649–654. [PubMed] [Google Scholar]
- Kyritsis A., Tsokos M., Chader G. Attachment culture of human retinoblastoma cells: long-term culture conditions and effects of dibutyryl cyclic AMP. Exp Eye Res. 1984 Apr;38(4):411–421. doi: 10.1016/0014-4835(84)90196-9. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Mills J. W. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979 Oct;83(1):159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T., Bighouse K. J. Correlation of rhodopsin biogenesis with ultrastructural morphogenesis in the chick retina. J Cell Biol. 1975 Jan;64(1):235–241. doi: 10.1083/jcb.64.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R., Winter J., Abney E. R., Pruss R. M., Gavrilovic J., Raff M. C. Myelin-specific proteins and glycolipids in rat Schwann cells and oligodendrocytes in culture. J Cell Biol. 1980 Mar;84(3):483–494. doi: 10.1083/jcb.84.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne N. N. Noradrenaline, a transmitter candidate in the retina. J Neurochem. 1981 Jan;36(1):17–27. doi: 10.1111/j.1471-4159.1981.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Palmer J. O., Kasselberg A. G., Netsky M. G. Differentiation of Medulloblastoma. Studies including immunohistochemical localization of glial fibrillary acidic protein. J Neurosurg. 1981 Aug;55(2):161–169. doi: 10.3171/jns.1981.55.2.0161. [DOI] [PubMed] [Google Scholar]
- Popoff N. A., Ellsworth R. M. The fine structure of retinoblastoma. In vivo and in vitro observations. Lab Invest. 1971 Nov;25(5):389–402. [PubMed] [Google Scholar]
- Prasad K. N. Differentiation of neuroblastoma cells in culture. Biol Rev Camb Philos Soc. 1975 May;50(2):129–165. doi: 10.1111/j.1469-185x.1975.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reid T. W., Albert D. M., Rabson A. S., Russell P., Craft J., Chu E. W., Tralka T. S., Wilcox J. L. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974 Aug;53(2):347–360. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- Schlesinger H. R., Rorke L., Jamieson R., Hummeler K. Neuronal properties of neuroectodermal tumors in vitro. Cancer Res. 1981 Jul;41(7):2573–2575. [PubMed] [Google Scholar]
- Stefansson K., Molnar M. L., Marton L. S., Molnar G. K., Mihovilovic M., Tripathi R. C., Richman D. P. Myelin-associated glycoprotein in human retina. Nature. 1984 Feb 9;307(5951):548–550. doi: 10.1038/307548a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Fisher S. K., Anderson D. H. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980 Apr 1;190(3):501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Itoyama Y., Kies M. W., Webster H. D. Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation. Proc Natl Acad Sci U S A. 1978 May;75(5):2521–2524. doi: 10.1073/pnas.75.5.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkkanen A., Tervo T., Tervo K., Eränkö L., Eränkö O., Cuello A. C. Substance P immunoreactivity in normal human retina and in retinoblastoma. Ophthalmic Res. 1983;15(6):300–306. doi: 10.1159/000265276. [DOI] [PubMed] [Google Scholar]
- Triche T. J., Askin F. B. Neuroblastoma and the differential diagnosis of small-, round-, blue-cell tumors. Hum Pathol. 1983 Jul;14(7):569–595. doi: 10.1016/s0046-8177(83)80202-0. [DOI] [PubMed] [Google Scholar]
- Ts'o M. O., Fine B. S., Zimmerman L. E. The nature of retinoblastoma. II. Photoreceptor differentiation: an electron microscopic study. Am J Ophthalmol. 1970 Mar;69(3):350–359. doi: 10.1016/0002-9394(70)92264-6. [DOI] [PubMed] [Google Scholar]
- Tso M. O. Clues to the cells of origin in retinoblastoma. Int Ophthalmol Clin. 1980 Summer;20(2):191–210. [PubMed] [Google Scholar]
- Tsokos M., Ross R. A., Triche T. J. Neuronal, Schwannian and melanocytic differentiation of human neuroblastoma cells in vitro. Prog Clin Biol Res. 1985;175:55–68. [PubMed] [Google Scholar]
- Uga S., Nakao F., Mimura M., Ikui H. Some new findings on the fine structure of the human photoreceptor cells. J Electron Microsc (Tokyo) 1970;19(1):71–84. [PubMed] [Google Scholar]