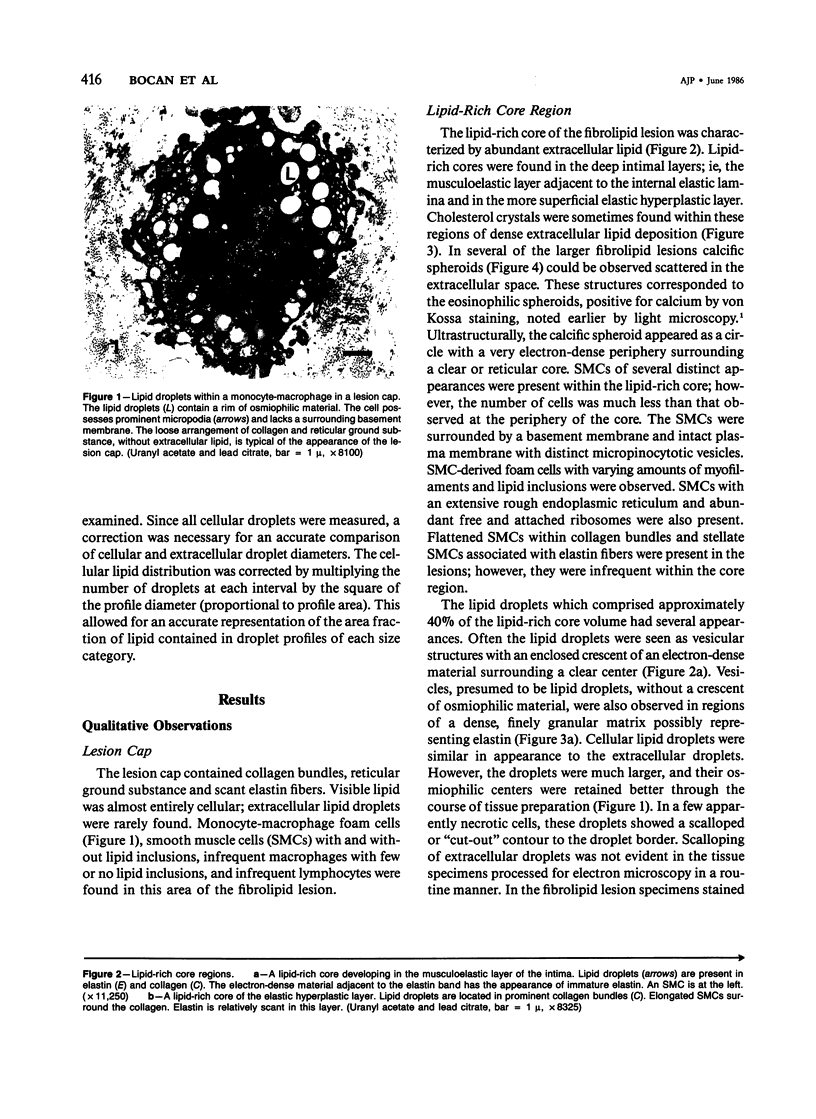
Abstract
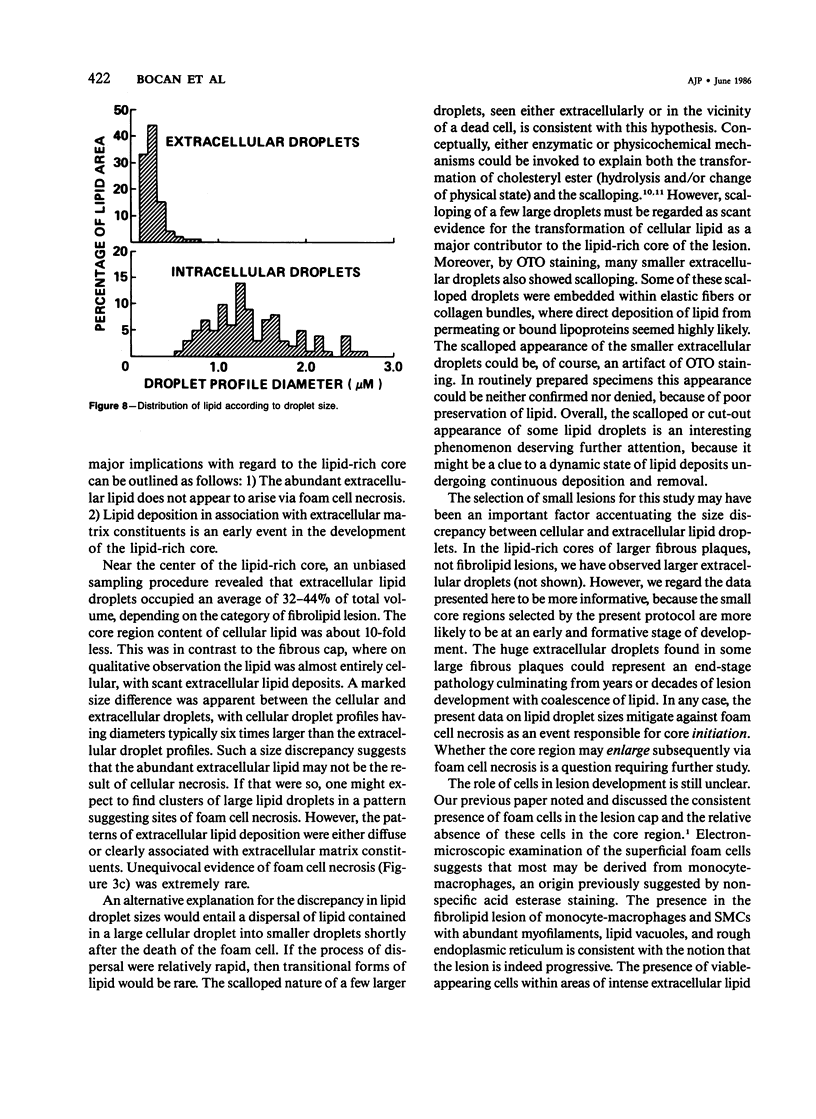
The formation of the atherosclerotic lipid-rich core has been elucidated by electron microscopy of the core region in small, raised fibrolipid lesions. The relationship among lipid deposits, extracellular matrix, and cells found in distinct regions of the fibrolipid lesion was examined. Extracellular lipid droplets, verified by osmium-thiocarbohydrazide-osmium staining, made up approximately 40% of the lipid-rich core volume. The lipid droplets were often found distinctly associated with elastin and/or collagen; these associations were dependent upon the location examined within or near the lipid-rich core. Within areas of intense extracellular lipid deposits, crystalline clefts suggesting cholesterol monohydrate were observed. Stereologic analysis of the lipid-rich core components revealed marked reductions in the volume fractions of cells, reticular ground substance, and basement membrane; while the extent of extracellular lipid increased 7-10-fold. Eleven percent or less of lipid in the core region was found within cells, usually smooth muscle cells. Above the core region in the lesion cap, monocyte-macrophage foam cells were prominent. Cellular lipid droplets were much larger (profile diameters sixfold higher) than extracellular droplets. With these data as well as transitional morphologic features at the boundaries of the core region, it is suggested that the abundant extracellular lipid does not derive from cell necrosis, and lipid deposition in association with extracellular matrix constituents is an early event in the development of the lipid-rich core.
Full text
PDF


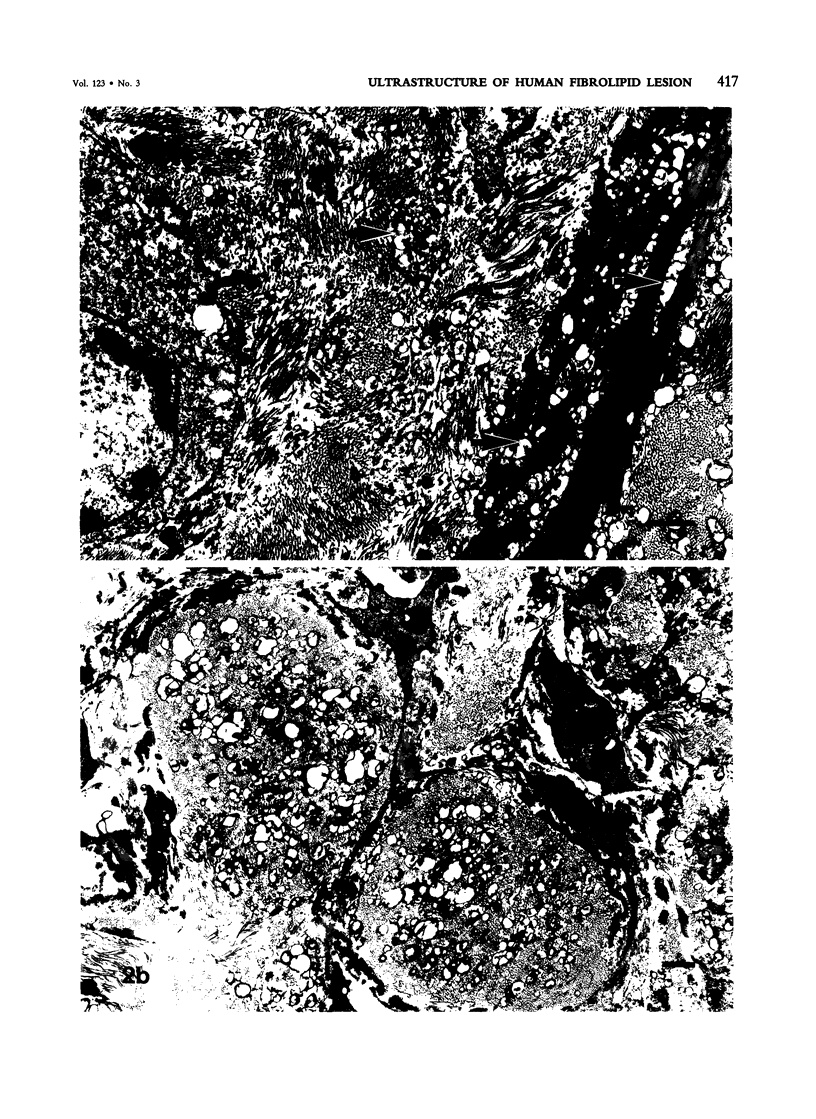


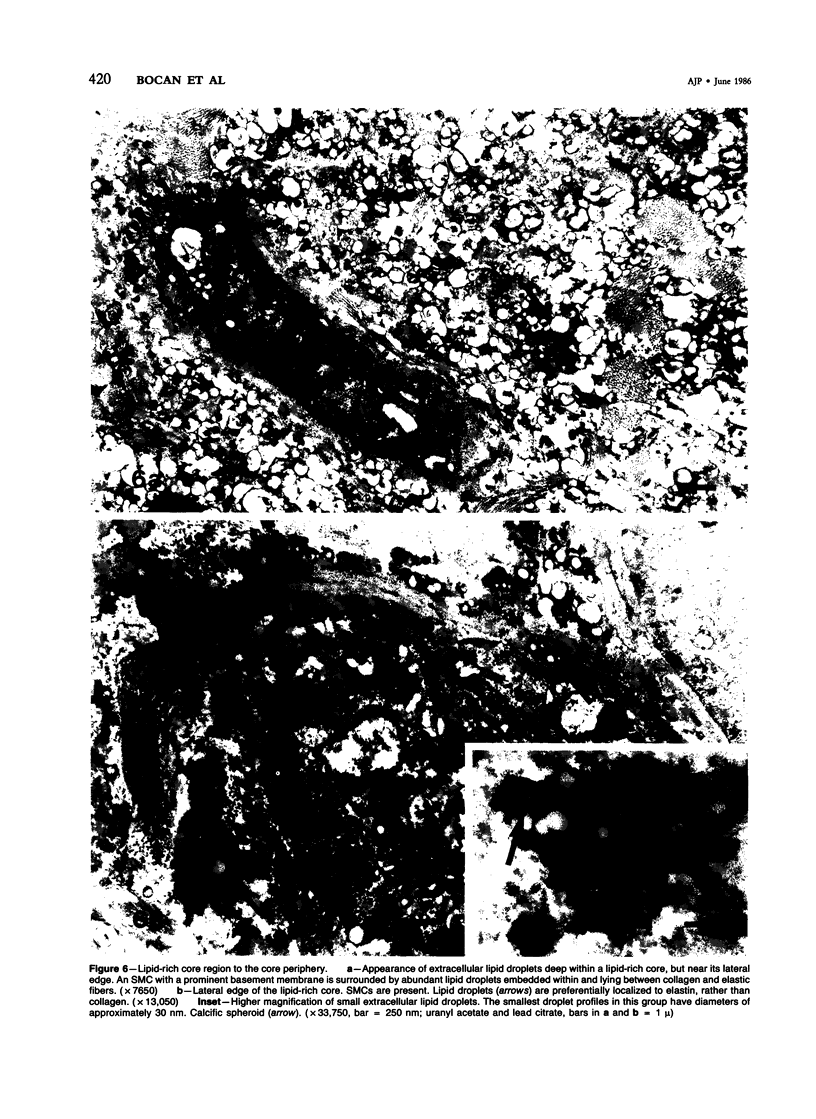



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Abdulla Y. H. The action of human high density lipoprotein on cholesterol crystals. Part 1. Light-microscopic observations. Atherosclerosis. 1978 Dec;31(4):465–471. doi: 10.1016/0021-9150(78)90142-9. [DOI] [PubMed] [Google Scholar]
- BALIS J. U., HAUST M. D., MORE R. H. ELECTRON-MICROSCOPIC STUDIES IN HUMAN ATHEROSCLEROSIS; CELLULAR ELEMENTS IN AORTIC FATTY STREAKS. Exp Mol Pathol. 1964 Oct;90:511–525. doi: 10.1016/0014-4800(64)90031-0. [DOI] [PubMed] [Google Scholar]
- Bocan T. M., Guyton J. R. Human aortic fibrolipid lesions. Progenitor lesions for fibrous plaques, exhibiting early formation of the cholesterol-rich core. Am J Pathol. 1985 Aug;120(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- GEER J. C., McGILL H. C., Jr, STRONG J. P. The fine structure of human atherosclerotic lesions. Am J Pathol. 1961 Mar;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- Guyton J. R., Bocan T. M., Schifani T. A. Quantitative ultrastructural analysis of perifibrous lipid and its association with elastin in nonatherosclerotic human aorta. Arteriosclerosis. 1985 Nov-Dec;5(6):644–652. doi: 10.1161/01.atv.5.6.644. [DOI] [PubMed] [Google Scholar]
- Haust M. D. The morphogenesis and fate of potential and early atherosclerotic lesions in man. Hum Pathol. 1971 Mar;2(1):1–29. doi: 10.1016/s0046-8177(71)80019-9. [DOI] [PubMed] [Google Scholar]
- Kramsch D. M., Hollander W. The interaction of serum and arterial lipoproteins with elastin of the arterial intima and its role in the lipid accumulation in atherosclerotic plaques. J Clin Invest. 1973 Feb;52(2):236–247. doi: 10.1172/JCI107180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu L., Avila E. M., Camejo G., León V., Liscano N. The structural stability of low-density lipoprotein. A kinetic X-ray scattering study of its interaction with arterial proteoglycans. Biochim Biophys Acta. 1984 Oct 4;795(3):525–534. doi: 10.1016/0005-2760(84)90182-6. [DOI] [PubMed] [Google Scholar]
- McGookey D. J., Anderson R. G. Morphological characterization of the cholesteryl ester cycle in cultured mouse macrophage foam cells. J Cell Biol. 1983 Oct;97(4):1156–1168. doi: 10.1083/jcb.97.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Takahashi T., Wada T. Elastin-lipid interaction in the arterial wall. Part 2. In vitro binding of lipoprotein-lipids to arterial elastin and the inhibitory effect of high density lipoproteins on the process. Atherosclerosis. 1981 Feb-Mar;38(3-4):373–382. doi: 10.1016/0021-9150(81)90053-8. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Smith E. B., Evans P. H., Downham M. D. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res. 1967 Mar-Apr;7(2):171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Walton K. W., Williamson N. Histological and immunofluorescent studies on the evolution of the human atheromatous plaque. J Atheroscler Res. 1968 Jul-Aug;8(4):599–624. doi: 10.1016/s0368-1319(68)80020-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Rutherford A. V. The use of osmium-thiocarbohydrazide-osmium (OTO) and ferrocyanide-reduced osmium methods to enhance membrane contrast and preservation in cultured cells. J Histochem Cytochem. 1984 Apr;32(4):455–460. doi: 10.1177/32.4.6323574. [DOI] [PubMed] [Google Scholar]