Abstract
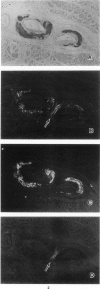
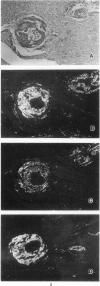
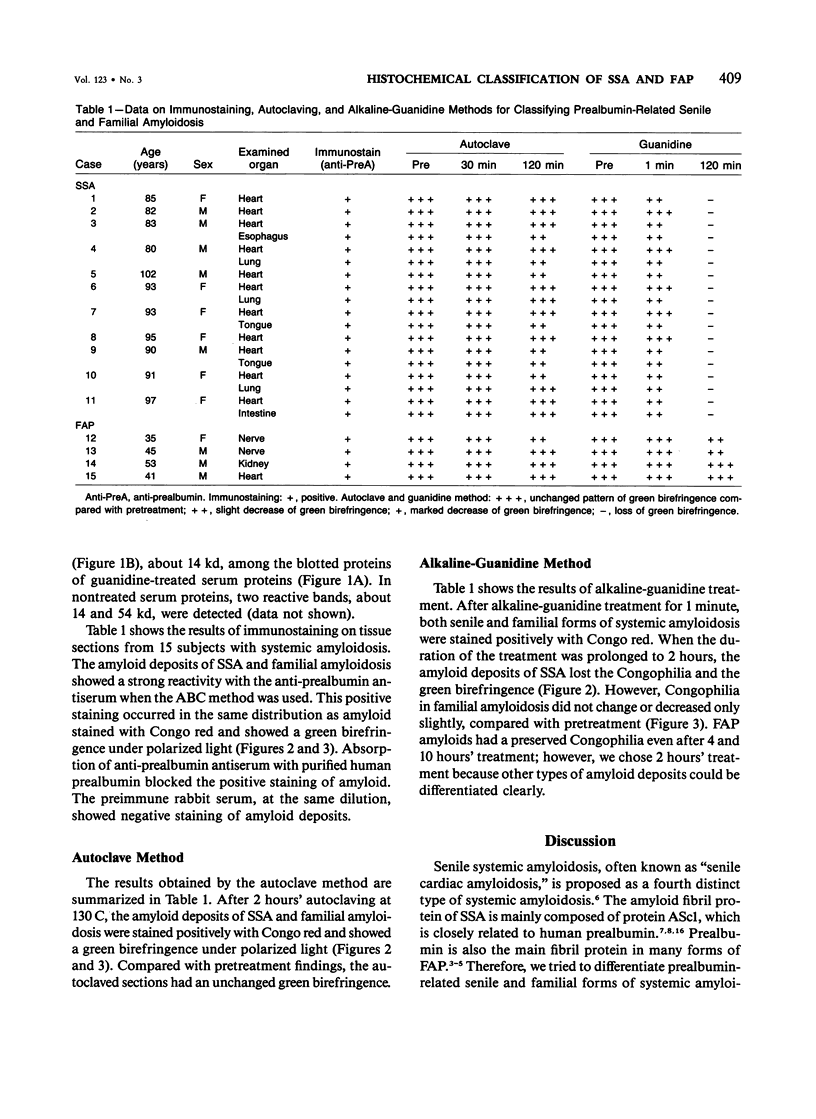
The immunoperoxidase method, the autoclave method, and a newly developed alkaline-guanidine method were used to distinguish senile (SSA) and familial types (FAP) of prealbumin-related amyloidosis in formalin-fixed, paraffin-embedded tissue sections. Because all the amyloid deposits of SSA and FAP reacted positively with the antiprealbumin antiserum, a classification of the amyloid fibril proteins of FAP and SSA by immunohistochemistry, using polyclonal anti-prealbumin antisera, was not feasible. Both the senile and familial forms of amyloidosis showed unchanged Congophilia after prolonged autoclaving. In the alkaline-guanidine method, FAP amyloids were resistant to incubation for 2 hours. On the other hand, amyloid deposits of SSA lost the Congophilia and green birefringence with 2 hours' alkaline-guanidine treatment. Therefore, the autoclave method combined with the alkaline-guanidine method will considerably facilitate differentiation of SSA and FAP, without specific antisera.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Mawatari S., Ohta M., Nakajima A., Kuroiwa Y. Polyneuritic amyloidosis in a Japanese family. Arch Neurol. 1968 Jun;18(6):593–602. doi: 10.1001/archneur.1968.00470360015001. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Shirahama T., Sipe J. D., Skinner M. Amyloid proteins, precursors, mediator, and enhancer. Lab Invest. 1983 Jan;48(1):1–4. [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Westermark P., Natvig J. B., Murdoch W. Senile cardiac amyloid: evidence that fibrils contain a protein immunologically related to prealbumin. Immunology. 1981 Nov;44(3):447–452. [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. C., Engel W. K. Amyloid in hereditary amyloid polyneuropathy is related to prealbumin. Arch Neurol. 1981 Jul;38(7):420–422. doi: 10.1001/archneur.1981.00510070054008. [DOI] [PubMed] [Google Scholar]
- DeLellis R. A., Glenner G. G., Ram J. S. Histochemical observations on amyloid with reference to polarization microscopy. J Histochem Cytochem. 1968 Oct;16(10):663–665. doi: 10.1177/16.10.663-a. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):694–698. doi: 10.1073/pnas.81.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding P., Fex G., Westermark P., Olofsson B. O., Pitkänen P., Benson L. Prealbumin in Swedish patients with senile systemic amyloidosis and familial amyloidotic polyneuropathy. Scand J Immunol. 1985 Feb;21(2):133–140. doi: 10.1111/j.1365-3083.1985.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Fex G., Laurell C. B., Thulin E. Purification of prealbumin from human and canine serum using a two-step affinity chromatographic procedure. Eur J Biochem. 1977 May 2;75(1):181–186. doi: 10.1111/j.1432-1033.1977.tb11515.x. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Harada M., Isersky C. The purification of amyloid fibril proteins. Prep Biochem. 1972;2(1):39–51. doi: 10.1080/00327487208061451. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Hikita K., Nagara H., Takeshita I. A new method to classify amyloid fibril proteins. Acta Neuropathol. 1985;67(3-4):272–278. doi: 10.1007/BF00687812. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ogata J., Okayama M., Goto I., Inomata H., Yoshida I., Omae T. Primary familial amyloidosis with vitreous opacities. Report of an autopsy case. Acta Neuropathol. 1978 Apr 26;42(1):67–70. doi: 10.1007/BF01273271. [DOI] [PubMed] [Google Scholar]
- Okayama M., Goto I., Ogata J., Omae T., Yoshida I., Inomata H. Primary amyloidosis with familial vitreous opacities: an unusual case and family. Arch Intern Med. 1978 Jan;138(1):105–111. [PubMed] [Google Scholar]
- Pitkänen P., Westermark P., Cornwell G. G., 3rd Senile systemic amyloidosis. Am J Pathol. 1984 Dec;117(3):391–399. [PMC free article] [PubMed] [Google Scholar]
- Rask L., Peterson P. A., Nilsson S. F. The subunit structure of human thyroxine-binding prealbumin. J Biol Chem. 1971 Oct 10;246(19):6087–6097. [PubMed] [Google Scholar]
- Sletten K., Westermark P., Natvig J. B. Senile cardiac amyloid is related to prealbumin. Scand J Immunol. 1980;12(6):503–506. doi: 10.1111/j.1365-3083.1980.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Tawara S., Araki S., Toshimori K., Nakagawa H., Ohtaki S. Amyloid fibril protein in type I familial amyloidotic polyneuropathy in Japanese. J Lab Clin Med. 1981 Dec;98(6):811–822. [PubMed] [Google Scholar]
- Tawara S., Nakazato M., Kangawa K., Matsuo H., Araki S. Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun. 1983 Nov 15;116(3):880–888. doi: 10.1016/s0006-291x(83)80224-1. [DOI] [PubMed] [Google Scholar]