Abstract
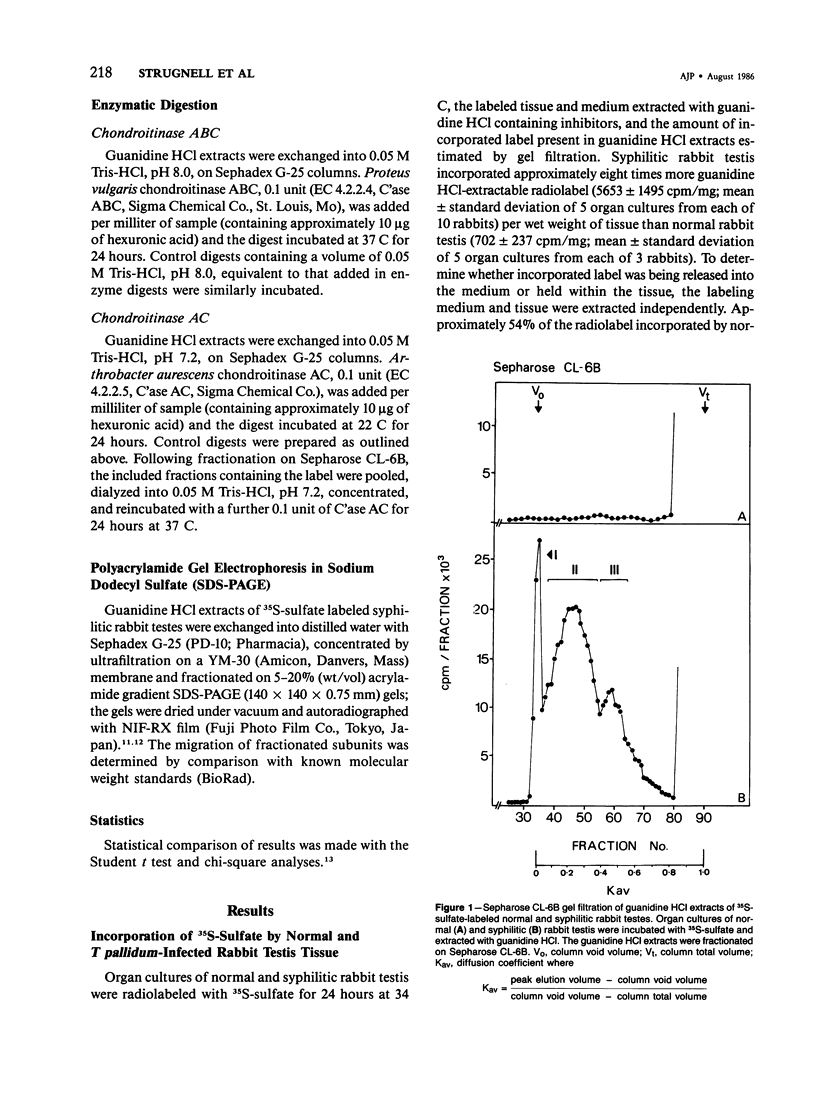
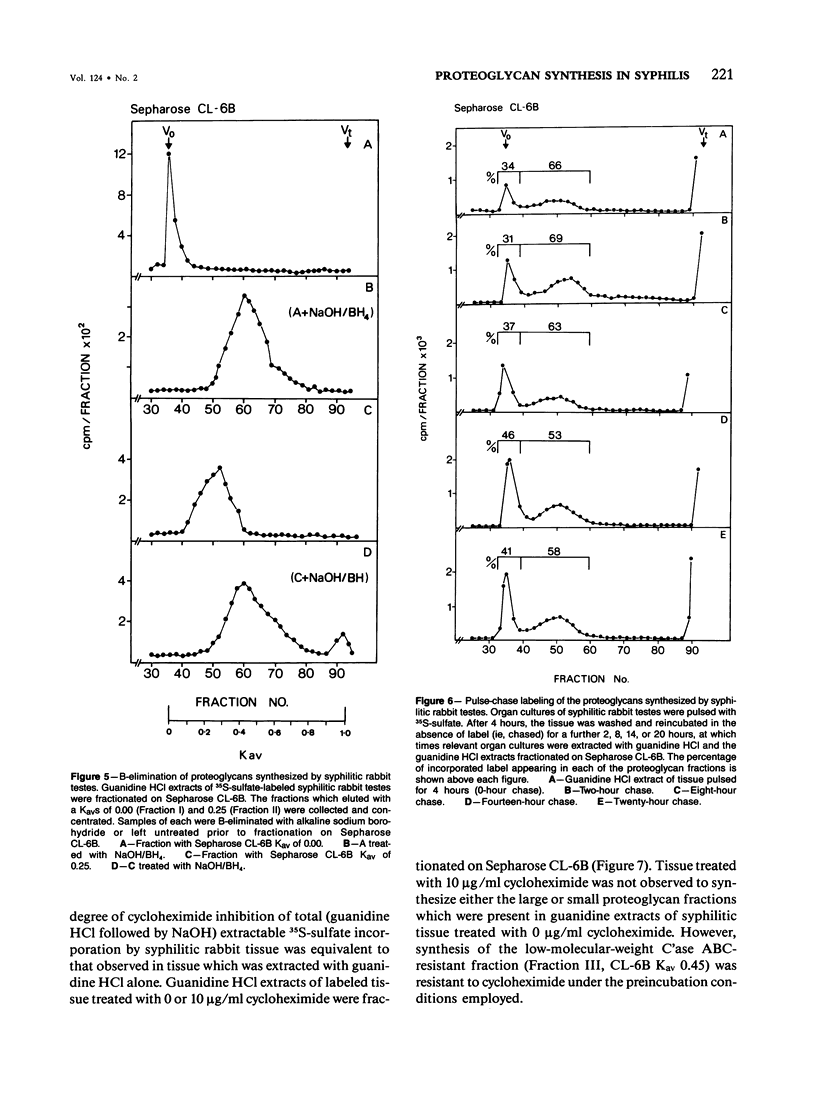
Organ cultures of syphilitic and normal rabbit testes were incubated with 35S-sulfate for labeling of proteoglycans. Syphilitic rabbit testes synthesized three macromolecular fractions (I, II, and III) which were not detected in extracts of normal uninfected tissue. The three fractions comprised a larger (approximately 10(6) mol wt) chondroitin sulfate/dermatan sulfate proteoglycan (Fraction I), a smaller (approximately 10(5) mol wt) chondroitin sulfate/dermatan sulfate proteoglycan (Fraction II), and a putative sulfated glycoprotein of Mr 40 kd (Fraction III). The glycosaminoglycan chains of both proteoglycans eluted with a Kav of 0.45 on Sepharose CL-6B, consistent with a molecular weight of 25,000. The smaller proteoglycan was not a cleavage product of the larger species. Erythromycin had no significant effect on the synthesis of any of the three macromolecules. In contrast, the synthesis of both proteoglycans was totally inhibited by a 2-hour preincubation with cycloheximide, which suggests that the constitutive "pools" of the two core proteins were small. The putative sulfated 40-kd glycoprotein was insensitive to a 2-hour preincubation with cycloheximide.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Fosang A. J., Handley C. J., Santer V., Lowther D. A., Thorburn G. D. Pregnancy-related changes in the connective tissue of the ovine cervix. Biol Reprod. 1984 Jun;30(5):1223–1235. doi: 10.1095/biolreprod30.5.1223. [DOI] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C., Brooks P. R., Lowther D. A. The relation of protein synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1984 Dec 15;224(3):977–988. doi: 10.1042/bj2240977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE F. A. Chondroitin sulfate and hyaluronic acid in syphilomas of cortisone-treated rabbits. Science. 1956 Aug 10;124(3215):275–275. doi: 10.1126/science.124.3215.275. [DOI] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Hyaluronidase and experimental syphilis. III. Metachromasia in syphilitic orchitis and its relationship to hyaluronic acid. Am J Syph Gonorrhea Vener Dis. 1950 Nov;34(6):501–514. [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M. Radiolabeling of Treponema pallidum (Nichols virulent strain) in vitro with precursors for protein and RNA biosynthesis. Infect Immun. 1978 Oct;22(1):22–28. doi: 10.1128/iai.22.1.22-28.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S., Powell H. C. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- Stevens R. L., Nissley S. P., Kimura J. H., Rechler M. M., Caplan A. I., Hascall V. C. Effects of insulin and multiplication-stimulating activity on proteoglycan biosynthesis in chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Feb 25;256(4):2045–2052. [PubMed] [Google Scholar]
- Strugnell R. A., Handley C. J., Drummond L., Faine S., Lowther D. A., Graves S. R. Polyanions in syphilis: evidence that glycoproteins and macromolecules resembling glycosaminoglycans are synthesised by host tissues in response to infection with Treponema pallidum. Br J Vener Dis. 1984 Apr;60(2):75–82. doi: 10.1136/sti.60.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugnell R. A., Handley C. J., Lowther D. A., Faine S., Graves S. R. Treponema pallidum does not synthesise in vitro a capsule containing glycosaminoglycans or proteoglycans. Br J Vener Dis. 1984 Feb;60(1):8–13. doi: 10.1136/sti.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Wrzolkowa T., Kozakiewicz J. Ultrastructure of vascular and connective tissue changes in primary syphilis. Br J Vener Dis. 1980 Jun;56(3):137–143. doi: 10.1136/sti.56.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Characterization of low buoyant density dermatan sulfate proteoglycans synthesized by rat ovarian granulosa cells in culture. J Biol Chem. 1983 Nov 10;258(21):12847–12856. [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis J. J., van Dijk G., Boer M., Stolz E., van Joost T. Mucopolysaccharides in suspensions of Treponema pallidum extracted from infected rabbit testes. Genitourin Med. 1985 Feb;61(1):7–12. doi: 10.1136/sti.61.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]



