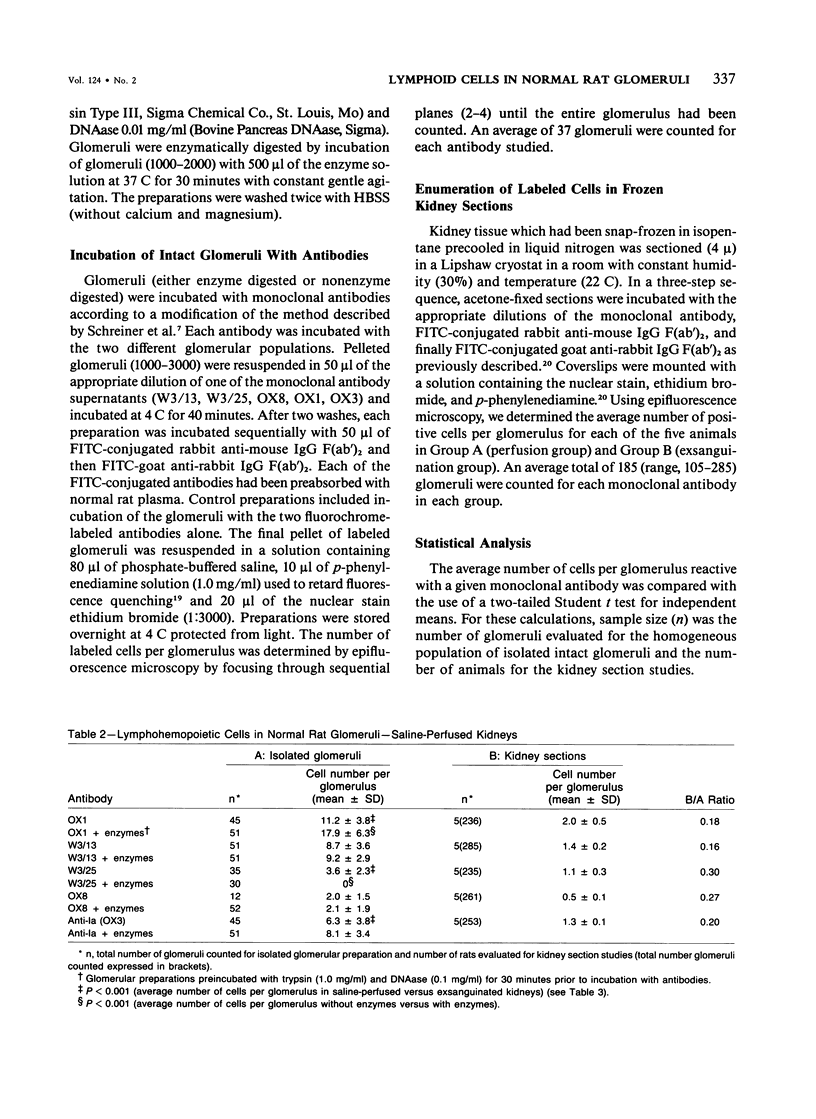
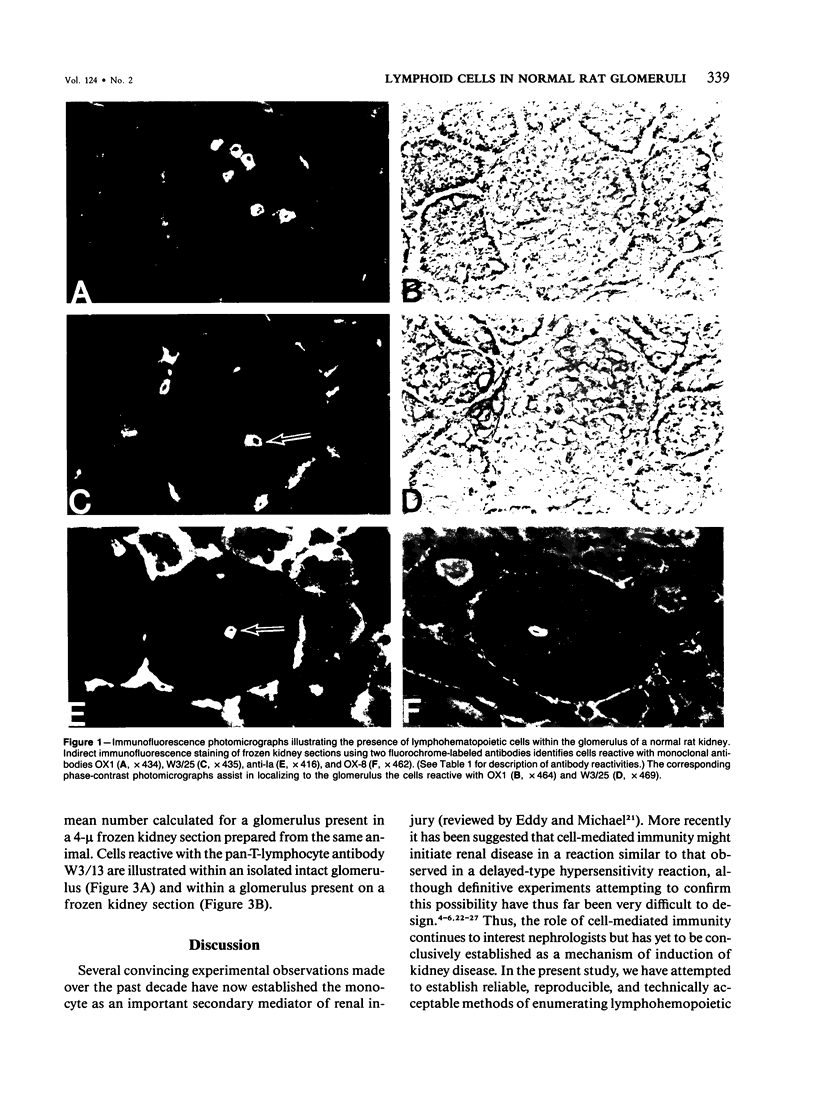
Abstract
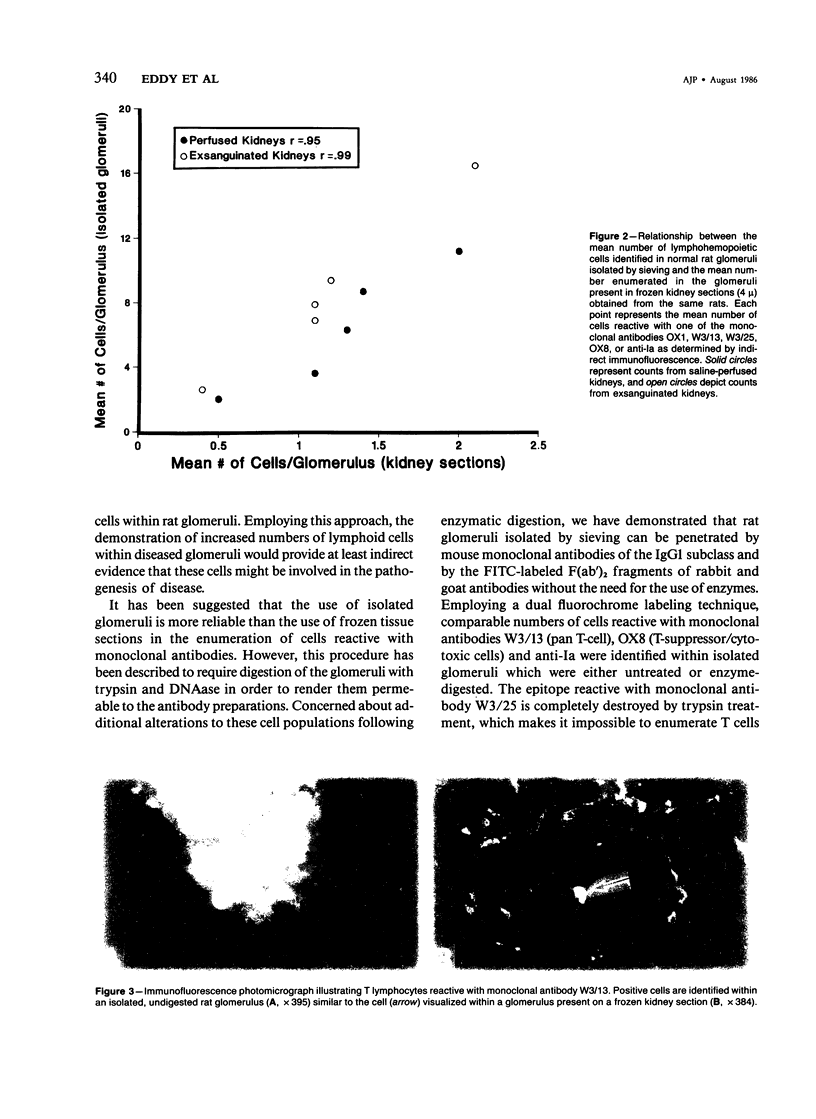
Recently developed monoclonal antibodies against rat lymphohematopoietic cells provide ideal probes for study of the role of the cellular immune system in experimental renal disease. Techniques for optimal and reliable labeling of cells present within the glomeruli have not yet been established. In this study it is shown that a small number of lymphoid and mononuclear cells can be identified within normal rat glomeruli present on frozen kidney sections (4 mu) when indirectly stained with monoclonal antibody W3/13, W3/25, OX1, OX3, or OX8 with the use of sequential incubations with F(ab')2 fragments of 2 fluorochrome-labeled antibodies, the nuclear stain ethidium bromide, and p-phenylenediamine added to retard fluorescence quenching. Cell counts showed good correlation with those obtained with the use of intact glomeruli isolated simultaneously from the same kidneys (r = 0.95 for saline-perfused kidneys; r = 0.99 for exsanguinated kidneys). Studies using isolated glomeruli pretreated with trypsin and DNAase failed to provide any advantage, because the enzymes did not enhance cellular reactivity with W3/13, OX8, or OX3, whereas the W3/25-reactive epitope was completely destroyed. It was only the OX1-reactive epitope which was enhanced by enzyme pretreatment. Thus, the described technique can accurately quantitate glomerular lymphohemopoietic cells on sections of frozen kidney and should provide a reliable method for the study of renal disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Collins A. B., Schneeberger E. E., McCluskey R. T. A cell-mediated reaction against glomerular-bound immune complexes. J Exp Med. 1979 Dec 1;150(6):1410–1420. doi: 10.1084/jem.150.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Collins A. B., McCluskey R. T. Evidence for a pathogenic role of a cell-mediated immune mechanism in experimental glomerulonephritis. J Exp Med. 1978 Jul 1;148(1):246–260. doi: 10.1084/jem.148.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Collins A. B., McCluskey R. T. Systemic cell-mediated reactions in vivo. Effect of the interaction of circulating antigen with sensitized lymphocytes on glomeruli and pulmonary alveoli. Am J Pathol. 1984 Jul;116(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Bohman S. O., Maunsbach A. B. Effects on tissue fine structure of variations in colloid osmotic pressure of glutaraldehyde fixatives. J Ultrastruct Res. 1970 Jan;30(1):195–208. doi: 10.1016/s0022-5320(70)90073-0. [DOI] [PubMed] [Google Scholar]
- Bolton W. K., Benton F. R., Lobo P. I. Requirement of functional T-cells in the production of autoimmune glomerulotubular nephropathy in mice. Clin Exp Immunol. 1978 Sep;33(3):474–477. [PMC free article] [PubMed] [Google Scholar]
- Bolton W. K., Tucker F. L., Sturgill B. C. New avian model of experimental glomerulonephritis consistent with mediation by cellular immunity. Nonhumorally mediated glomerulonephritis in chickens. J Clin Invest. 1984 May;73(5):1263–1276. doi: 10.1172/JCI111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Eddy A., Newman S. L., Cosio F., LeBien T., Michael A. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984 Jun;31(3):371–389. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- Garber S. L., O'Morchoe P. J., O'Morchoe C. C. Effect of experimental glomerulonephritis on the cells in canine renal lymph with special reference to the veiled cell. Clin Exp Immunol. 1982 Aug;49(2):347–354. [PMC free article] [PubMed] [Google Scholar]
- Green J. R. Generation of cytotoxic T cells in the rat mixed lymphocyte reaction is blocked by monoclonal antibody MRC OX-8. Immunology. 1984 Jun;52(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R. Fc dependence of macrophage accumulation and subsequent injury in experimental glomerulonephritis. J Immunol. 1983 Feb;130(2):735–739. [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Plattner S. B., Weisenburger D. The role of monocytes in serum sickness nephritis. J Exp Med. 1979 Sep 19;150(3):413–425. doi: 10.1084/jem.150.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Anversa P., Melissari M., Loud A. V. Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int. 1980 Apr;17(4):438–454. doi: 10.1038/ki.1980.52. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S. Localization of an Ia-bearing glomerular cell in the mesangium. J Cell Biol. 1982 Aug;94(2):483–488. doi: 10.1083/jcb.94.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Kiely J. M., Cotran R. S., Unanue E. R. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest. 1981 Oct;68(4):920–931. doi: 10.1172/JCI110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Origin of the rat mesangial phagocyte and its expression of the leukocyte common antigen. Lab Invest. 1984 Nov;51(5):515–523. [PubMed] [Google Scholar]
- Shea S. M., Morrison A. B. A stereological study of the glomerular filter in the rat. Morphometry of the slit diaphragm and basement membrane. J Cell Biol. 1975 Nov;67(2PT1):436–443. doi: 10.1083/jcb.67.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefoni S., Vangelista A., Nanni Costa A., Bonomini V. Short-term thoracic duct drainage in drug resistant immunologically mediated glomerulonephritis. Evaluation of lymph and blood lymphocyte characteristics during drainage. Clin Nephrol. 1981 Dec;16(6):300–306. [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Balderas R., Shawler D. L., Izui S., Kotzin B. L., Strober S., Dixon F. J. Inhibition of T cells proliferation and SLE-like syndrome of MRL/1 mice by whole body or total lymphoid irradiation. J Immunol. 1980 Nov;125(5):2137–2142. [PubMed] [Google Scholar]
- Thomas M. L., Green J. R. Molecular nature of the W3/25 and MRC OX-8 marker antigens for rat T lymphocytes: comparisons with mouse and human antigens. Eur J Immunol. 1983 Oct;13(10):855–858. doi: 10.1002/eji.1830131014. [DOI] [PubMed] [Google Scholar]
- Tipping P. G., Neale T. J., Holdsworth S. R. T lymphocyte participation in antibody-induced experimental glomerulonephritis. Kidney Int. 1985 Mar;27(3):530–537. doi: 10.1038/ki.1985.43. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]