Abstract
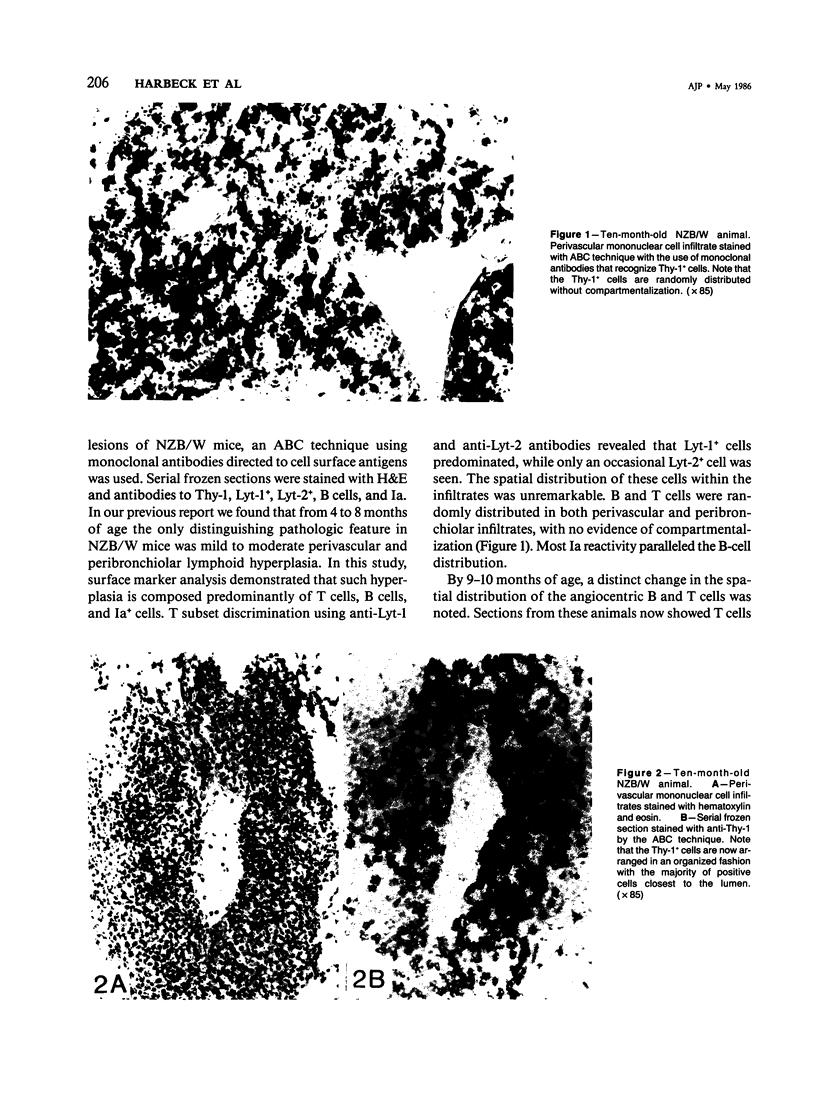
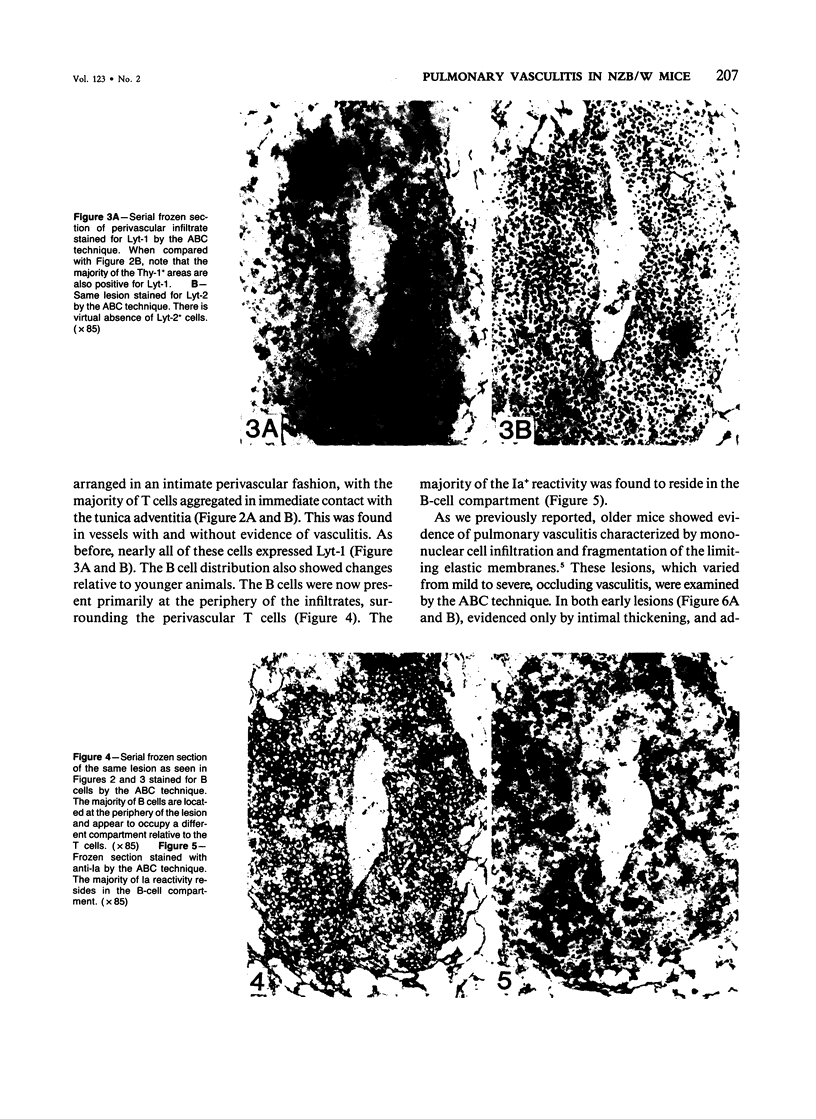
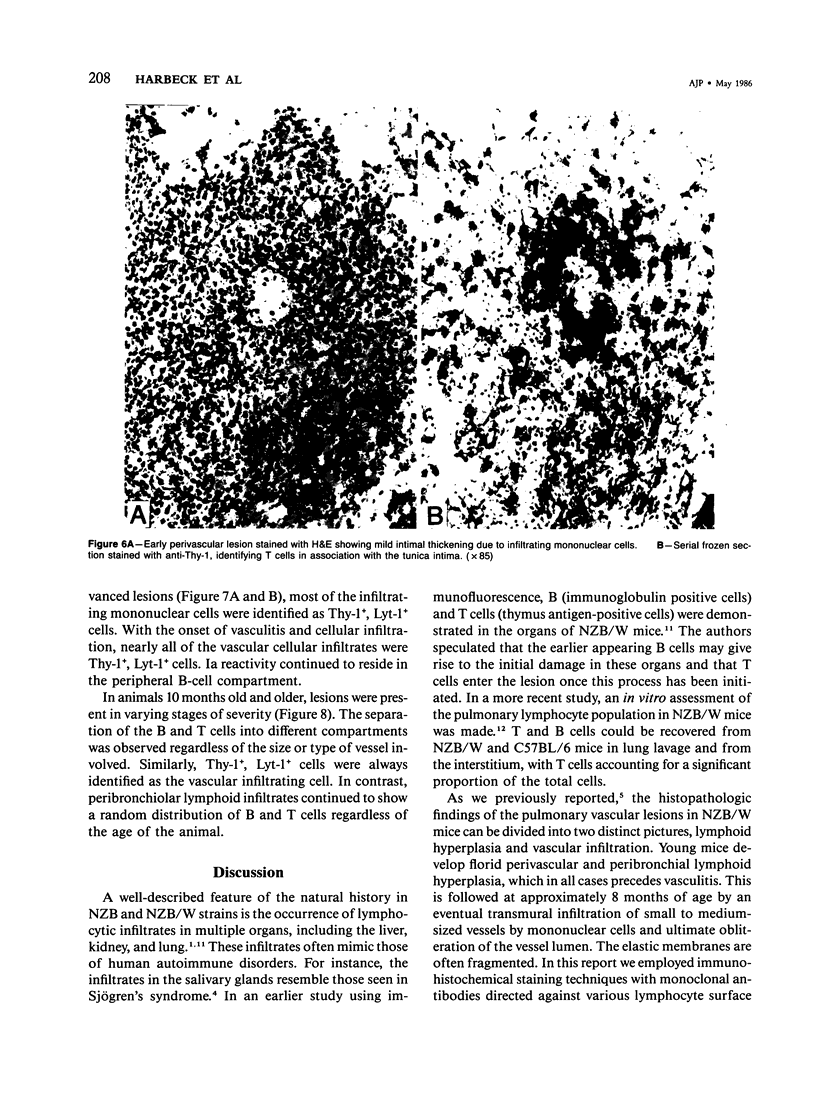
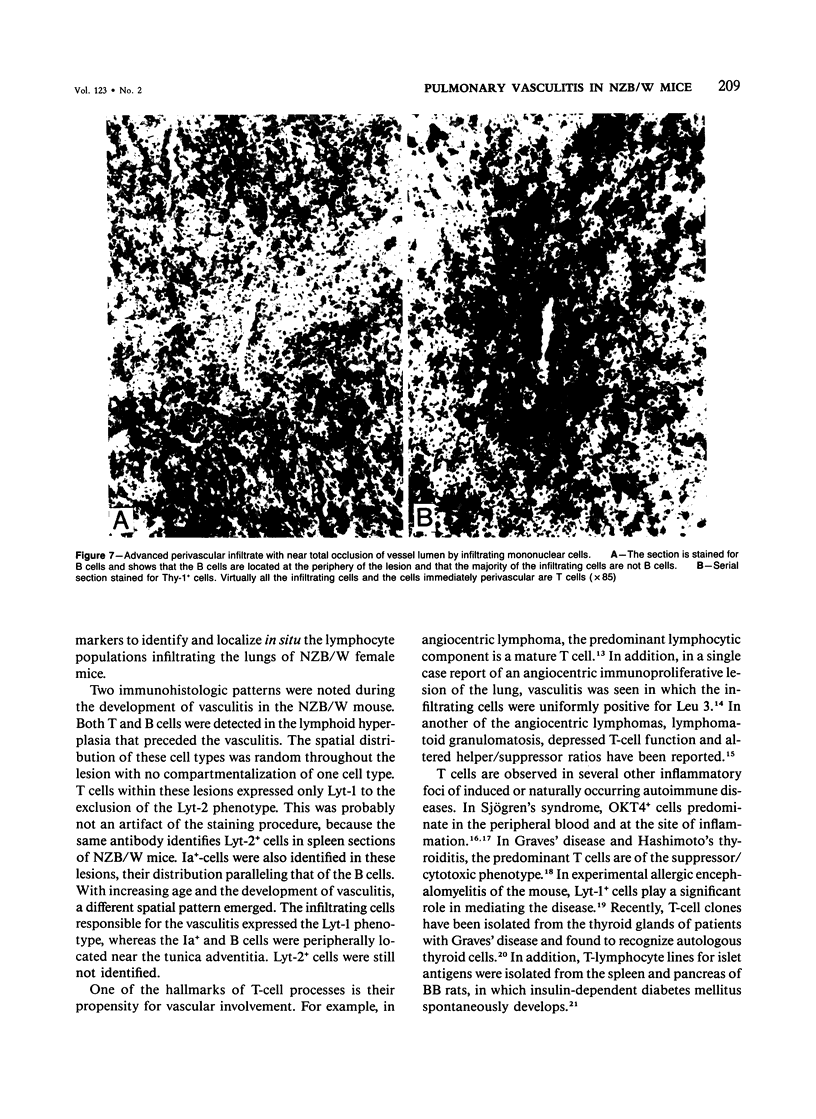
NZB/W mice spontaneously develop pulmonary lymphoid hyperplasia and vasculitis in an age-related fashion. The cellular infiltrates and pattern of involvement bear similarity to various forms of pulmonary vasculitis in humans. In this study the authors used monoclonal antibodies and the avidin-biotin immunoperoxidase technique to analyze the pulmonary mononuclear cell infiltrates of female NZB/W mice at various ages and levels of disease activity. T cells, T-cell subsets, B cells, and Ia-bearing cells were localized with this technique. Most cells within the infiltrates were T cells that expressed the Lyt-1 phenotype, whereas cells expressing Lyt-2 were rarely observed. Cells reacting with a monoclonal antibody which recognizes cells of the B-lineage and cells expressing Ia antigens were also observed. Before the development of vasculitis, B and T cells were randomly distributed throughout the lesion. In older animals with vasculitis, T cells expressing Lyt-1 were associated with vessel lumen and were primarily responsible for the vascular infiltration, to the apparent exclusion of other lymphoid cell types. The B cells and Ia+ cells were localized at the periphery of the lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Chan C. C., Mochizuki M., Nussenblatt R. B., Palestine A. G., McAllister C., Gery I., BenEzra D. T-lymphocyte subsets in experimental autoimmune uveitis. Clin Immunol Immunopathol. 1985 Apr;35(1):103–110. doi: 10.1016/0090-1229(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. A monoclonal antibody that recognizes B cells and B cell precursors in mice. J Exp Med. 1981 Feb 1;153(2):269–279. doi: 10.1084/jem.153.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Hyman R., Lesley J., Trowbridge I. S. Effects of cytotoxic monoclonal antibody specific for T200 glycoprotein on functional lymphoid cell populations. Cell Immunol. 1980 Aug 1;53(2):350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Carstens S. A., Fong S., Robinson C. A., Howell F., Vaughan J. H. Use of monoclonal antibodies to analyze peripheral blood and salivary gland lymphocyte subsets in Sjögren's syndrome. Arthritis Rheum. 1982 Apr;25(4):419–426. doi: 10.1002/art.1780250410. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Gong H., Jr, Clements P. J., Eisenberg H. Pulmonary lymphocyte subpopulations. Variations in New Zealand black/white and C57BL/6 mice with age. Am Rev Respir Dis. 1979 Oct;120(4):821–827. doi: 10.1164/arrd.1979.120.4.821. [DOI] [PubMed] [Google Scholar]
- Greenspan J. S., Gutman G. A., Weissman I. L., Talal N. Thymus-antigen- and immunoglobulin-positive lymphocytes in tissue infiltrates of NZB/NZW mice. Clin Immunol Immunopathol. 1974 Sep;3(1):16–31. doi: 10.1016/0090-1229(74)90020-8. [DOI] [PubMed] [Google Scholar]
- Hauser S. L., Weiner H. L., Bhan A. K., Shapiro M. E., Che M., Aldrich W. R., Letvin N. L. Lyt-1 cells mediate acute murine experimental allergic encephalomyelitis. J Immunol. 1984 Nov;133(5):2288–2290. [PubMed] [Google Scholar]
- Hoffman R. W., Alspaugh M. A., Waggie K. S., Durham J. B., Walker S. E. Sjögren's syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984 Feb;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Yamanaka N., Ogawa K., Yoshida Y., Takami T., Matsuura A., Isago H., Kataura A., Kikuchi K. Nasal T-cell lymphoma as a type of so-called "lethal midline granuloma". Cancer. 1982 Dec 1;50(11):2336–2344. doi: 10.1002/1097-0142(19821201)50:11<2336::aid-cncr2820501120>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kessler H. S. A laboratory model for Sjögren's syndrome. Am J Pathol. 1968 Mar;52(3):671–685. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., Hogarth P. M., Ceredig R., McKenzie I. F. Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J Exp Med. 1981 May 1;153(5):1044–1057. doi: 10.1084/jem.153.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron R. M., Kempski O., Spatz M., McFarlin D. E. Presentation of myelin basic protein by murine cerebral vascular endothelial cells. J Immunol. 1985 May;134(5):3100–3103. [PubMed] [Google Scholar]
- Misaki T., Konishi J., Nakashima T., Iida Y., Kasagi K., Endo K., Uchiyama T., Kuma K., Torizuka K. Immunohistological phenotyping of thyroid infiltrating lymphocytes in Graves' disease and Hashimoto's thyroiditis. Clin Exp Immunol. 1985 Apr;60(1):104–110. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme G. J., Fieser T. M., Dixon F. J., Theofilopoulos A. N. B-cell-tropic interleukins in murine systemic lupus erythematosus (SLE) 1. Immunol Rev. 1984 Apr;78:159–183. doi: 10.1111/j.1600-065x.1984.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Prud'homme G. J., Fuks A., Colle E., Guttmann R. D. Isolation of T-lymphocyte lines with specificity for islet cell antigens from spontaneously diabetic (insulin-dependent) rats. Diabetes. 1984 Aug;33(8):801–803. doi: 10.2337/diab.33.8.801. [DOI] [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J Immunol. 1984 May;132(5):2402–2407. [PubMed] [Google Scholar]
- Sordillo P. P., Epremian B., Koziner B., Lacher M., Lieberman P. Lymphomatoid granulomatosis: an analysis of clinical and immunologic characteristics. Cancer. 1982 May 15;49(10):2070–2076. doi: 10.1002/1097-0142(19820515)49:10<2070::aid-cncr2820491019>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Staszak C., Harbeck R. J. Mononuclear-cell pulmonary vasculitis in NZB/W mice. I. Histopathologic evaluation of spontaneously occurring pulmonary infiltrates. Am J Pathol. 1985 Jul;120(1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Shawler D. L., Eisenberg R. A., Dixon F. J. Splenic immunoglobulin-secreting cells and their regulation in autoimmune mice. J Exp Med. 1980 Feb 1;151(2):446–466. doi: 10.1084/jem.151.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni K. K., Holley K. E., McDuffie F. C., Titus J. L. Comparative study of NZB mice under germfree and conventional conditions. J Rheumatol. 1975 Mar;2(1):36–44. [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., McKenzie I. F., Chism S. E., Shen F. W., Boyse E. A., Gamble J. R., Whitelaw A. M. Ly and Ia antigen phenotypes of T cells involved in delayed-type hypersensitivity and in suppression. J Exp Med. 1976 Jul 1;144(1):10–19. doi: 10.1084/jem.144.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., McKimm-Breschkin L., Harris A. W., Schrader J. W. Interferon-gamma induces enhanced expression of Ia and H-2 antigens on B lymphoid, macrophage, and myeloid cell lines. J Immunol. 1983 Aug;131(2):788–793. [PubMed] [Google Scholar]










