Abstract
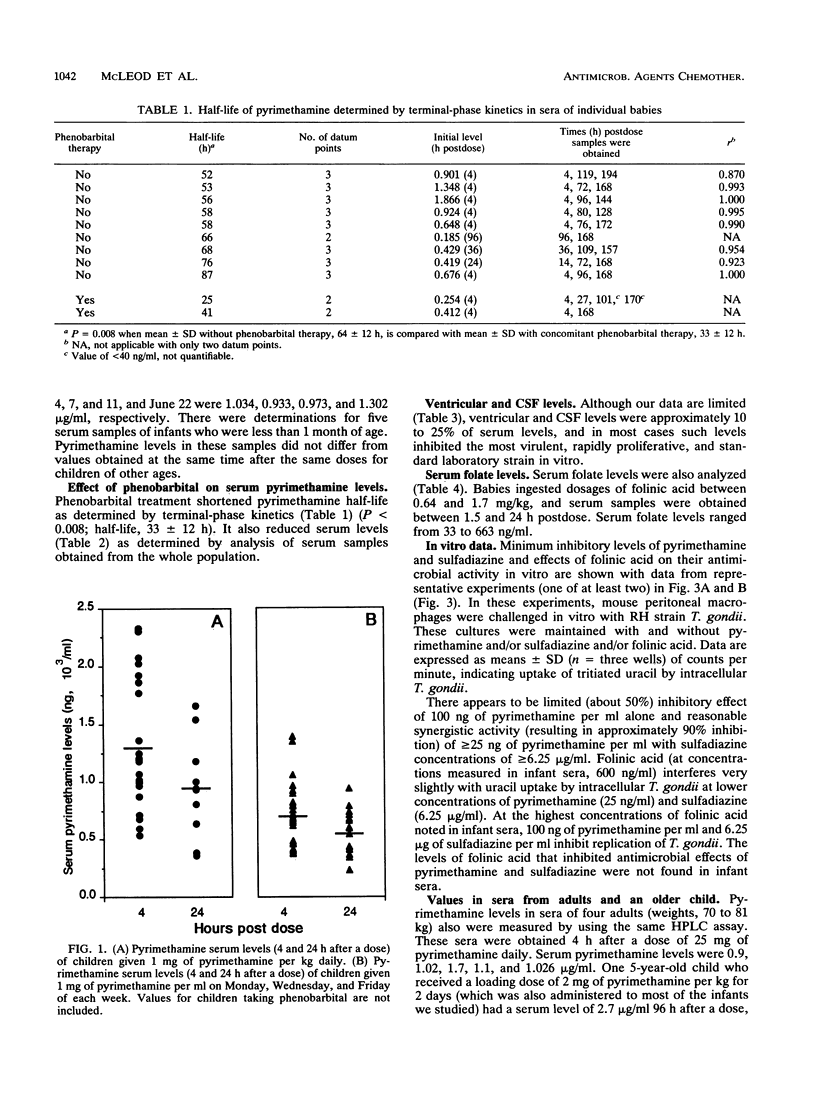
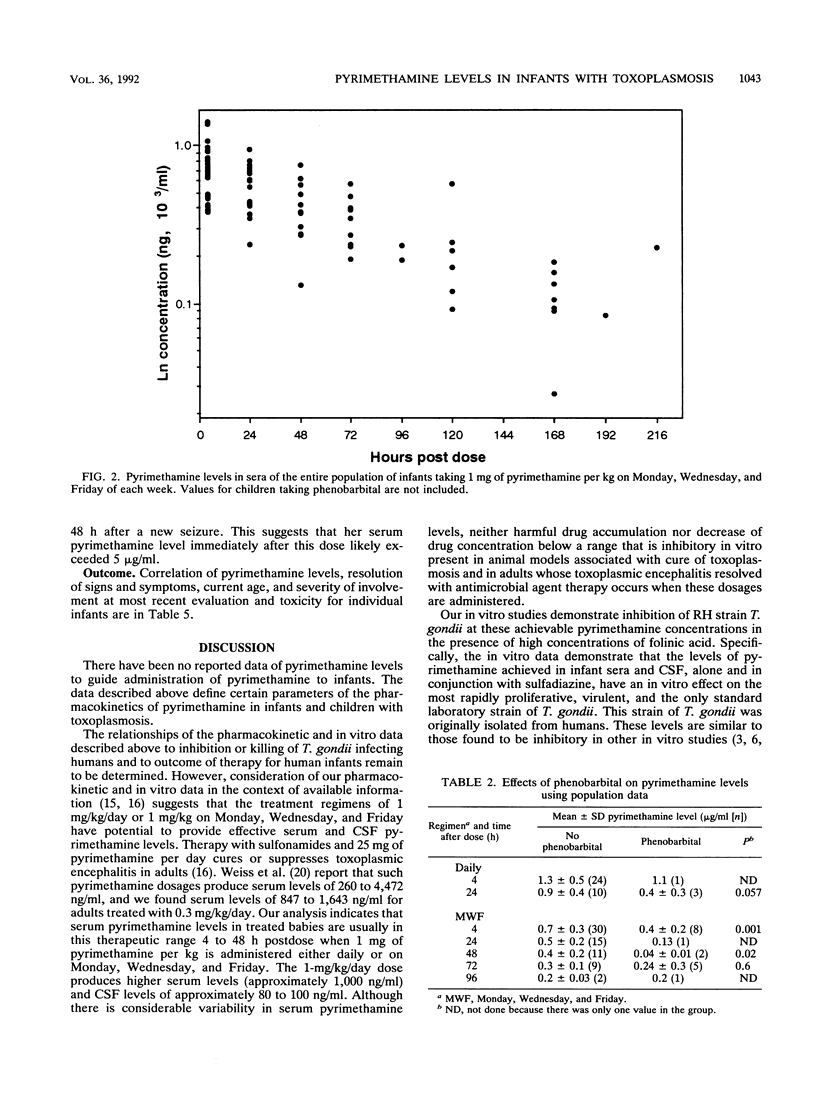
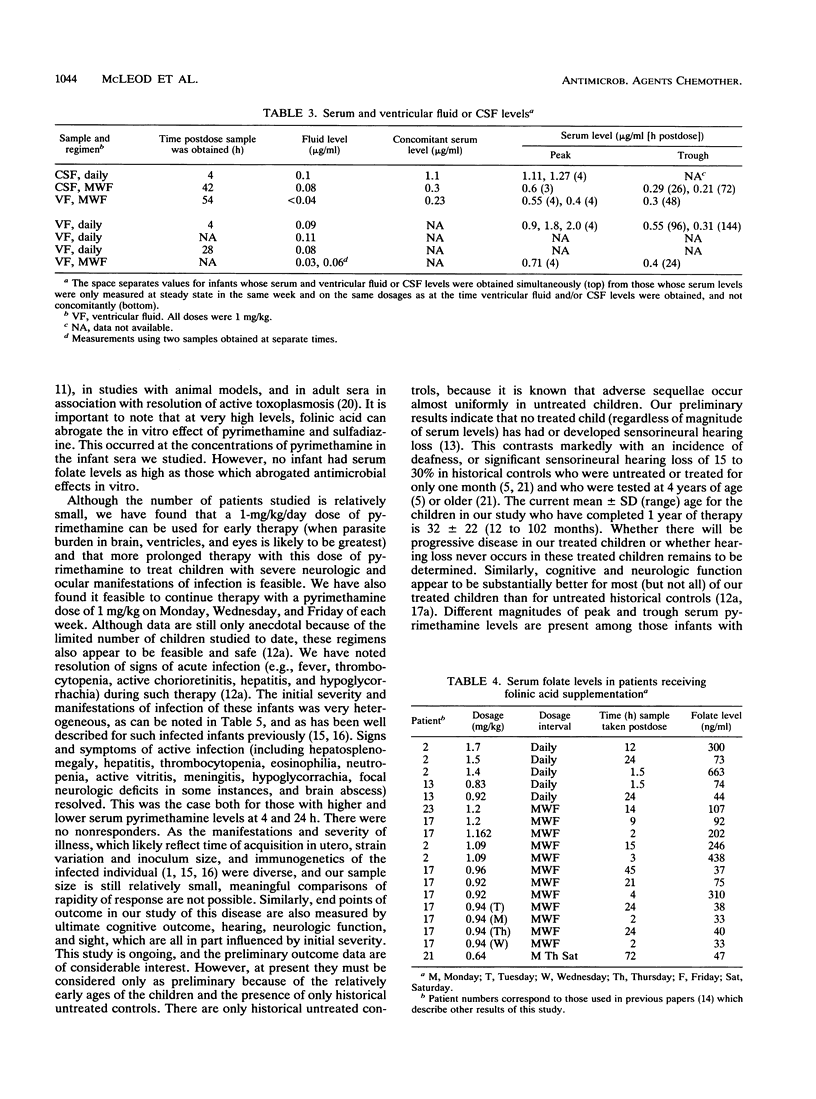
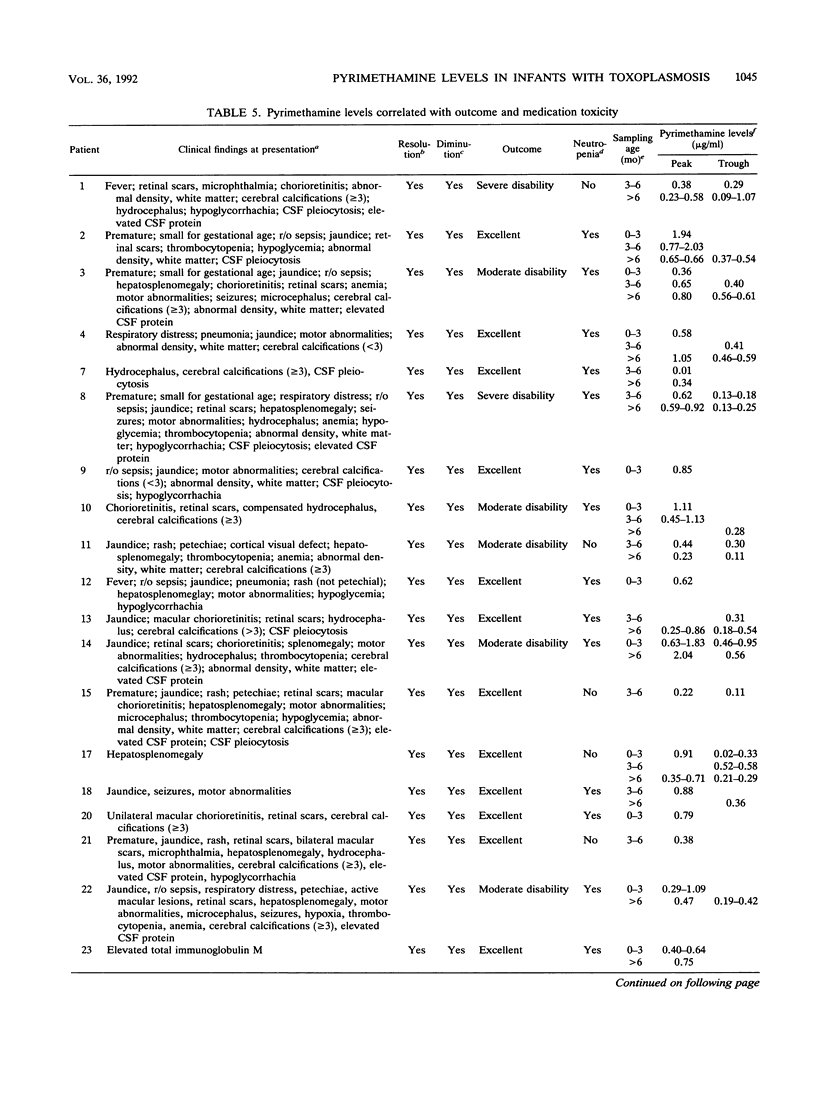
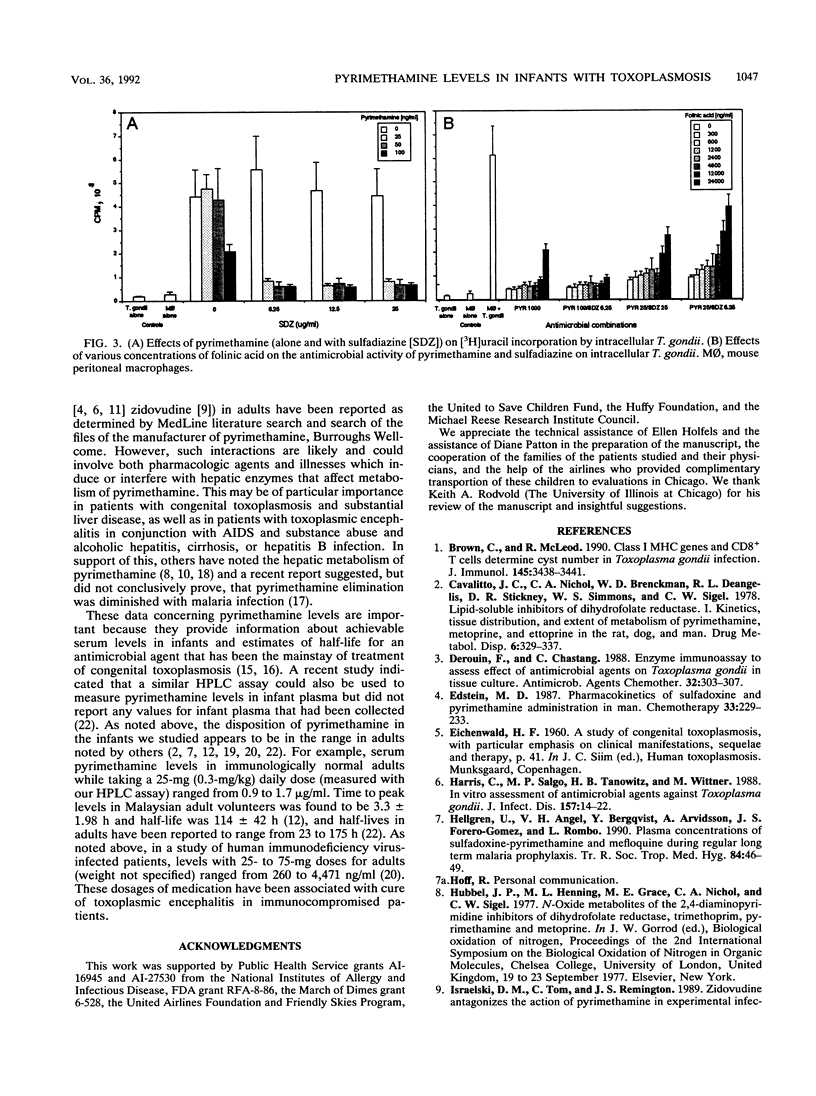
Pyrimethamine levels in sera, cerebrospinal fluid (CSF), and ventricular fluid were measured by using reversed-phase high-pressure liquid chromatography. The specimens were from 37 infants receiving pyrimethamine for treatment of suspect or proven congenital toxoplasmosis. Pyrimethamine half-life in serum was 64 +/- 12 h when determined by study of terminal-phase kinetics of samples obtained from nine babies. This half-life was significantly different (P = 0.008) from the pyrimethamine half-life (33 +/- 12 h) determined by terminal-phase kinetics for two babies of the same age taking phenobarbital. Serum pyrimethamine levels at various intervals after dosages of pyrimethamine were also lower for infants receiving phenobarbital. Levels measured in sera from babies taking the same dose of pyrimethamine throughout their first year of life did not appear to vary significantly over time or at different ages (P greater than 0.05). Mean +/- standard deviation serum levels 4 h after a pyrimethamine dose were 1.297 +/- 0.54 micrograms/ml for babies taking 1 mg of pyrimethamine per kg of body weight daily and 0.7 +/- 0.26 microgram/ml for babies taking 1 mg/kg each Monday, Wednesday, and Friday. Levels in CSF were approximately 10 to 25% of concomitant levels in serum. Serum folate levels for infants who took 0.64 to 1.7 mg leukovorin per kg ranged from 33 to 663 ng/ml. To determine whether the levels of pyrimethamine in serum and CSF of treated infants were in a range that affected the most virulent, rapidly replicating, and standard laboratory strain of Toxoplasma gondii, effects of various concentrations of pyrimethamine and sulfadiazine on replication of T. gondii in vitro were assessed. The levels of the antimicrobial agents effective in vitro were in the range of levels of pyrimethamine achieved in sera and CSF. Although folinic acid could inhibit the therapeutic effect of pyrimethamine and sulfadiazine in vitro, inhibition was noted only at levels (> or = 4,800 ng/ml) that were considerably higher than the folate levels found in the treated infants' sera.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown C. R., McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990 Nov 15;145(10):3438–3441. [PubMed] [Google Scholar]
- Cavallito J. C., Nichol C. A., Brenckman W. D., Jr, Deangelis R. L., Stickney D. R., Simmons W. S., Sigel C. W. Lipid-soluble inhibitors of dihydrofolate reductase. I. Kinetics, tissue distribution, and extent of metabolism of pyrimethamine, metoprine, and etoprine in the rat, dog, and man. Drug Metab Dispos. 1978 May-Jun;6(3):329–337. [PubMed] [Google Scholar]
- Derouin F., Chastang C. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrob Agents Chemother. 1988 Mar;32(3):303–307. doi: 10.1128/aac.32.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstein M. D. Pharmacokinetics of sulfadoxine and pyrimethamine after Fansidar administration in man. Chemotherapy. 1987;33(4):229–233. doi: 10.1159/000238499. [DOI] [PubMed] [Google Scholar]
- Harris C., Salgo M. P., Tanowitz H. B., Wittner M. In vitro assessment of antimicrobial agents against Toxoplasma gondii. J Infect Dis. 1988 Jan;157(1):14–22. doi: 10.1093/infdis/157.1.14. [DOI] [PubMed] [Google Scholar]
- Hellgren U., Angel V. H., Bergqvist Y., Arvidsson A., Forero-Gomez J. S., Rombo L. Plasma concentrations of sulfadoxine-pyrimethamine and of mefloquine during regular long term malaria prophylaxis. Trans R Soc Trop Med Hyg. 1990 Jan-Feb;84(1):46–49. doi: 10.1016/0035-9203(90)90376-p. [DOI] [PubMed] [Google Scholar]
- Kumar R., Kaushik P., Sharma C. B. Studies on the determination and degradation of pyrimethamine in mammals. Biomed Chromatogr. 1990 Jul;4(4):165–167. doi: 10.1002/bmc.1130040411. [DOI] [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansor S. M., Navaratnam V., Mohamad M., Hussein S., Kumar A., Jamaludin A., Wernsdorfer W. H. Single dose kinetic study of the triple combination mefloquine/sulphadoxine/pyrimethamine (Fansimef) in healthy male volunteers. Br J Clin Pharmacol. 1989 Mar;27(3):381–386. doi: 10.1111/j.1365-2125.1989.tb05381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T., Wolters C., Stein L., Kraus N., Johnson D., Boyer K., Mets M., Roizen N., Beckman J., Meier P. Absence of sensorineural hearing loss in treated infants and children with congenital toxoplasmosis. Otolaryngol Head Neck Surg. 1992 Jan;106(1):75–80. doi: 10.1177/019459989210600131. [DOI] [PubMed] [Google Scholar]
- McLeod R., Mack D. G., Boyer K., Mets M., Roizen N., Swisher C., Patel D., Beckmann E., Vitullo D., Johnson D. Phenotypes and functions of lymphocytes in congenital toxoplasmosis. J Lab Clin Med. 1990 Nov;116(5):623–635. [PubMed] [Google Scholar]
- Mihaly G. W., Date N. M., Veenendaal J. R., Newman K. T., Smallwood R. A. Decreased hepatic elimination of pyrimethamine during malaria infection. Studies in the isolated perfused rat liver. Biochem Pharmacol. 1987 Sep 1;36(17):2827–2829. doi: 10.1016/0006-2952(87)90272-3. [DOI] [PubMed] [Google Scholar]
- SCHMIDT L. H., HUGHES H. B., SCHMIDT I. G. The pharmacological properties of 2, 4-diamino-5-p-chlorophenyl-6-ethylpyrimidine, daraprim. J Pharmacol Exp Ther. 1953 Jan;107(1):92–130. [PubMed] [Google Scholar]
- Wang N. S., Guo X. B., Liu Q. D., Fu L. C., Li G. Q., Arnold K. Pharmacokinetics of the combination pyrimethamine with sulfadoxine and mefloquine (FANSIMEF) in Chinese volunteers and the relative bioavailability of a lacquered tablet. Chemotherapy. 1990;36(3):177–184. doi: 10.1159/000238764. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Harris C., Berger M., Tanowitz H. B., Wittner M. Pyrimethamine concentrations in serum and cerebrospinal fluid during treatment of acute Toxoplasma encephalitis in patients with AIDS. J Infect Dis. 1988 Mar;157(3):580–583. doi: 10.1093/infdis/157.3.580. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S., Stagno S., Reynolds D. W. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics. 1980 Nov;66(5):767–774. [PubMed] [Google Scholar]
- Zytkovicz T. H., Salter J., Hennigan L., Timperi R., Maguire J., Hoff R. Isocratic reversed-phase HPLC method to measure pyrimethamine extracted from plasma of infants treated for toxoplasmosis. Clin Chem. 1991 Jul;37(7):1281–1283. [PubMed] [Google Scholar]


