Abstract
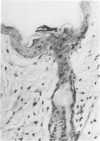
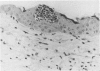
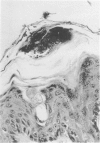
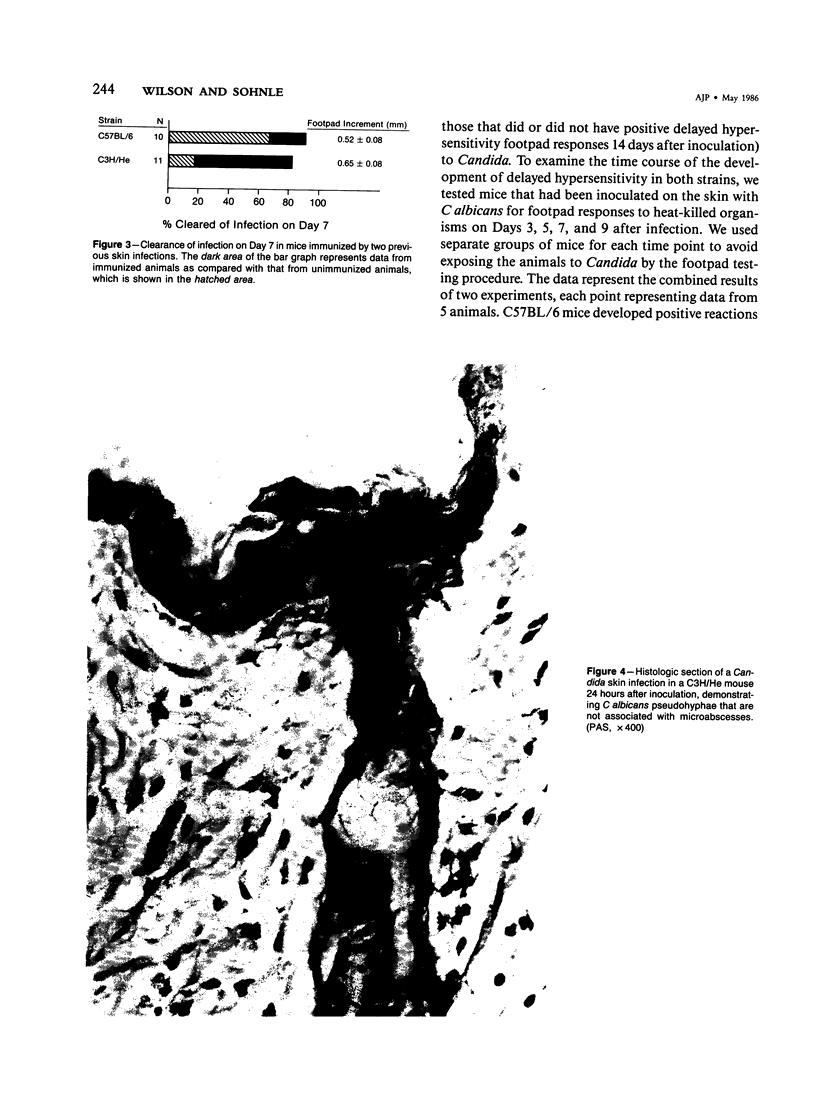
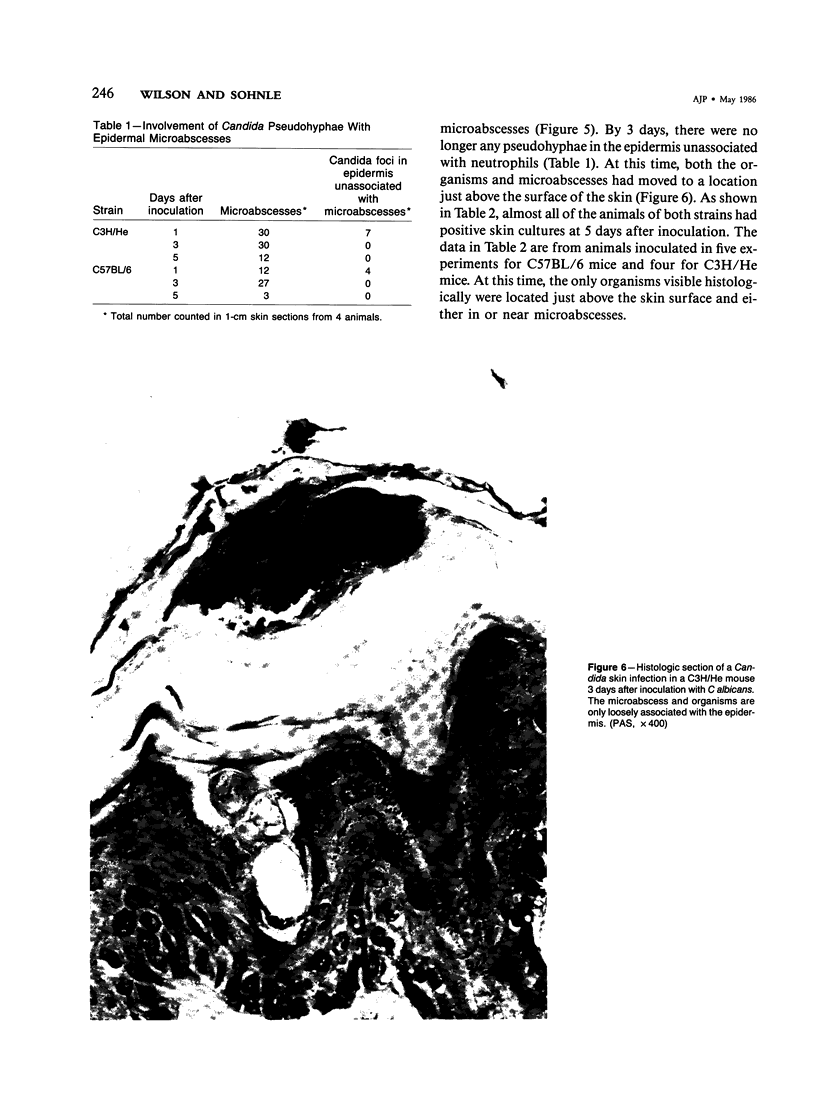
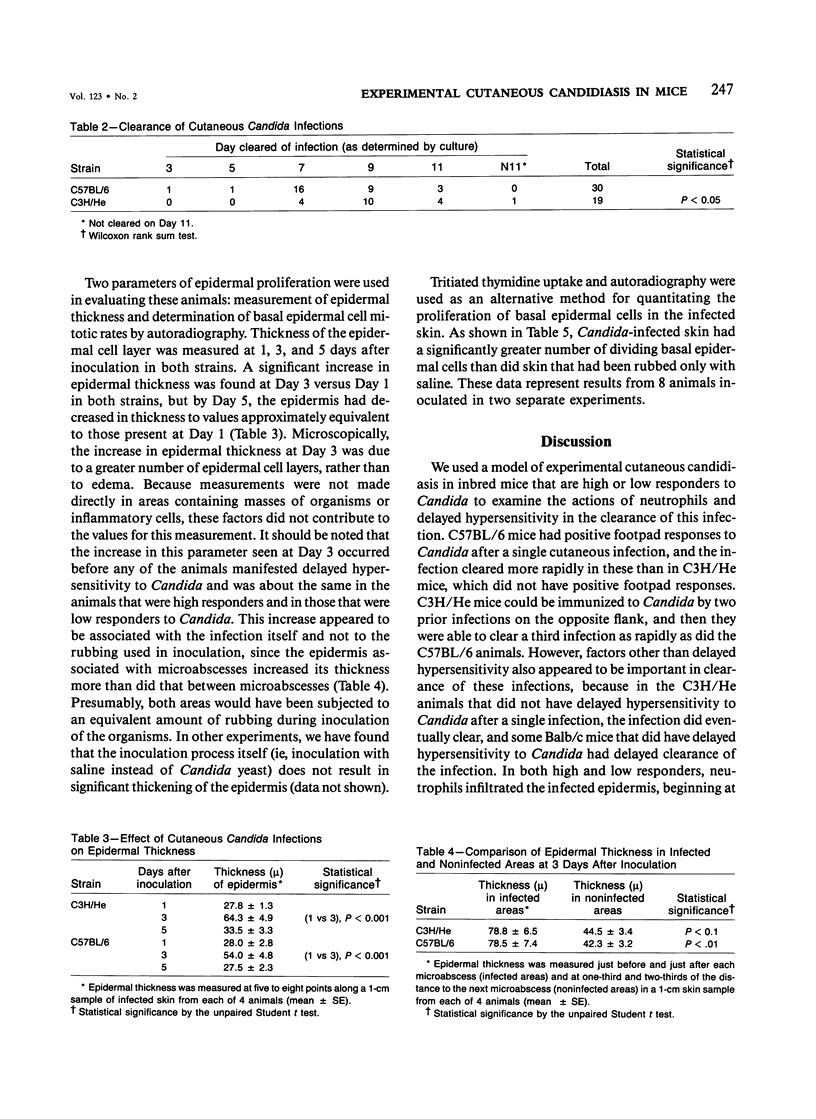
Involvement of neutrophils and delayed hypersensitivity in the clearance of Candida albicans infections was investigated with the use of a model of the disease in inbred mice. Experimental infections were produced by rubbing C albicans onto the shaved skin of the flank without the use of occlusive dressings. After a single infection, delayed hypersensitivity to Candida developed in C57BL/6 mice, and the infection cleared more rapidly than in C3H/He mice, in which delayed hypersensitivity did not develop. In both strains, the organisms were associated with neutrophilic microabscesses in the upper epidermis within 1 day of inoculation; by 3 days, the organisms and microabscesses had become relocated to a site just above the skin surface. At this time, the epidermis was intact under the microabscesses and significantly thickened, which indicated that epidermal proliferation had occurred. Delayed hypersensitivity reactions accelerated clearance of the infection, apparently by increasing the rate of removal of the microabscesses and associated organisms from the skin surface. However, delayed hypersensitivity was not an absolute requirement for clearance, because in animals of the C3H/He strain, in which delayed hypersensitivity did not develop during the first infection, the infection was eventually cleared. It is postulated that in these infections an important defense mechanism may be the enhancement, perhaps by the neutrophilic infiltrate, of epidermal proliferation early in the infection such that the infecting organisms are moved to a location above the skin surface from which they can be more easily removed by other processes, including delayed hypersensitivity reactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman C. A., Shea M. J., Frame P. T. Invasive fungal infections in patients with chronic mucocutaneous candidiasis. Arch Intern Med. 1981 Jul;141(8):1076–1079. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Smith T. K. Chronic mucocutaneous candidiasis: immunologic and antibiotic therapy. Ann Intern Med. 1974 Mar;80(3):310–320. doi: 10.7326/0003-4819-80-3-310. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E. Effects of cyclophosphamide on murine candidiasis. Infect Immun. 1980 Feb;27(2):376–386. doi: 10.1128/iai.27.2.376-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Sundsmo J. S., Weiser R. S. Lymphokine toxicity for yeast cells. J Immunol. 1973 May;110(5):1444–1446. [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Experimental cutaneous candidiasis in rodents; II. Role of the stratum corneum barrier and serum complement as a mediator of a protective infalmmatory response. Arch Dermatol. 1978 Apr;114(4):539–543. doi: 10.1001/archderm.114.4.539. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Rycroft R. J., Valdimarsson H., Bannister L. H., Wells R. S. Chronic muco-cutaneous candidiasis of late onset, thymoma and myopathy. A report of four cases. Clin Exp Dermatol. 1976 Mar;1(1):59–74. doi: 10.1111/j.1365-2230.1976.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Sauder D. N., Carter C. S., Katz S. I., Oppenheim J. J. Epidermal cell production of thymocyte activating factor (ETAF). J Invest Dermatol. 1982 Jul;79(1):34–39. doi: 10.1111/1523-1747.ep12510569. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Frank M. M., Kirkpatrick C. H. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976 Aug;117(2):523–530. [PubMed] [Google Scholar]
- Sohnle P. G., Kirkpatrick C. H. Epidermal proliferation in the defense against experimental cutaneous candidiasis. J Invest Dermatol. 1978 Mar;70(3):130–133. doi: 10.1111/1523-1747.ep12258536. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Kirkpatrick C. H. Study of possible mechanisms of basophil accumulation in experimental cutaneous candidiasis in guinea pigs. J Allergy Clin Immunol. 1977 Feb;59(2):171–177. doi: 10.1016/0091-6749(77)90221-4. [DOI] [PubMed] [Google Scholar]