Abstract
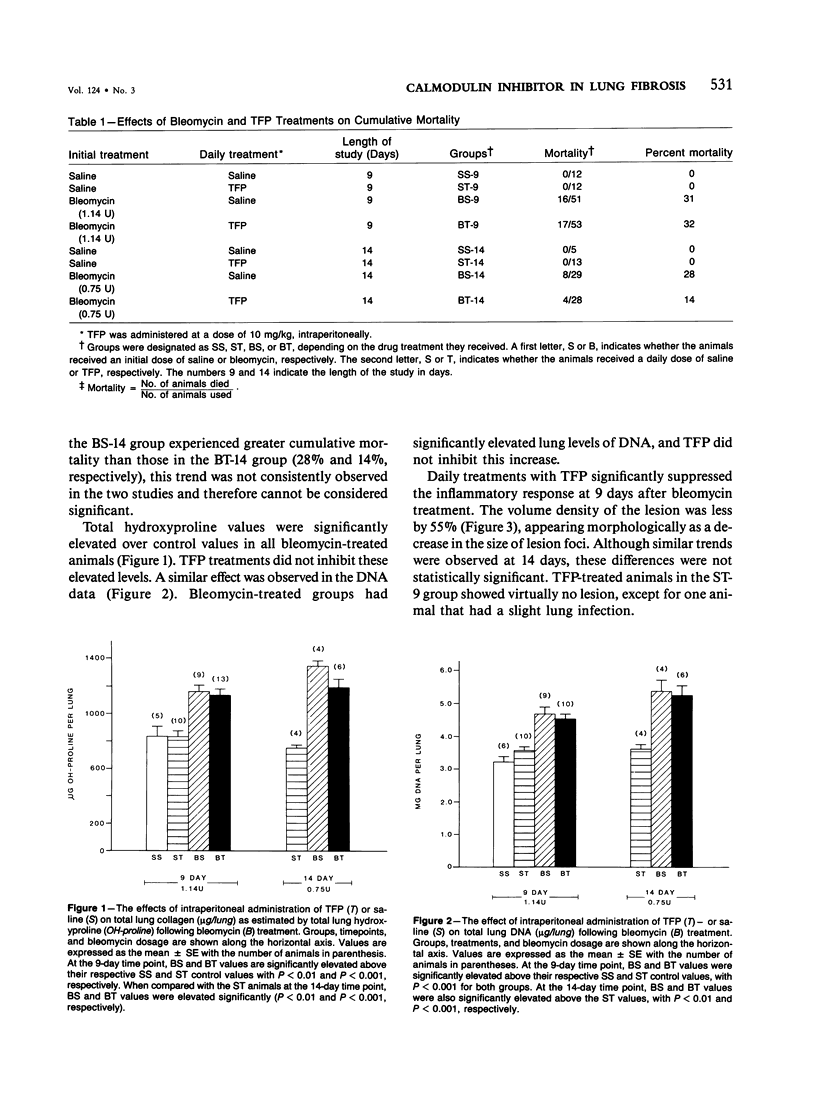
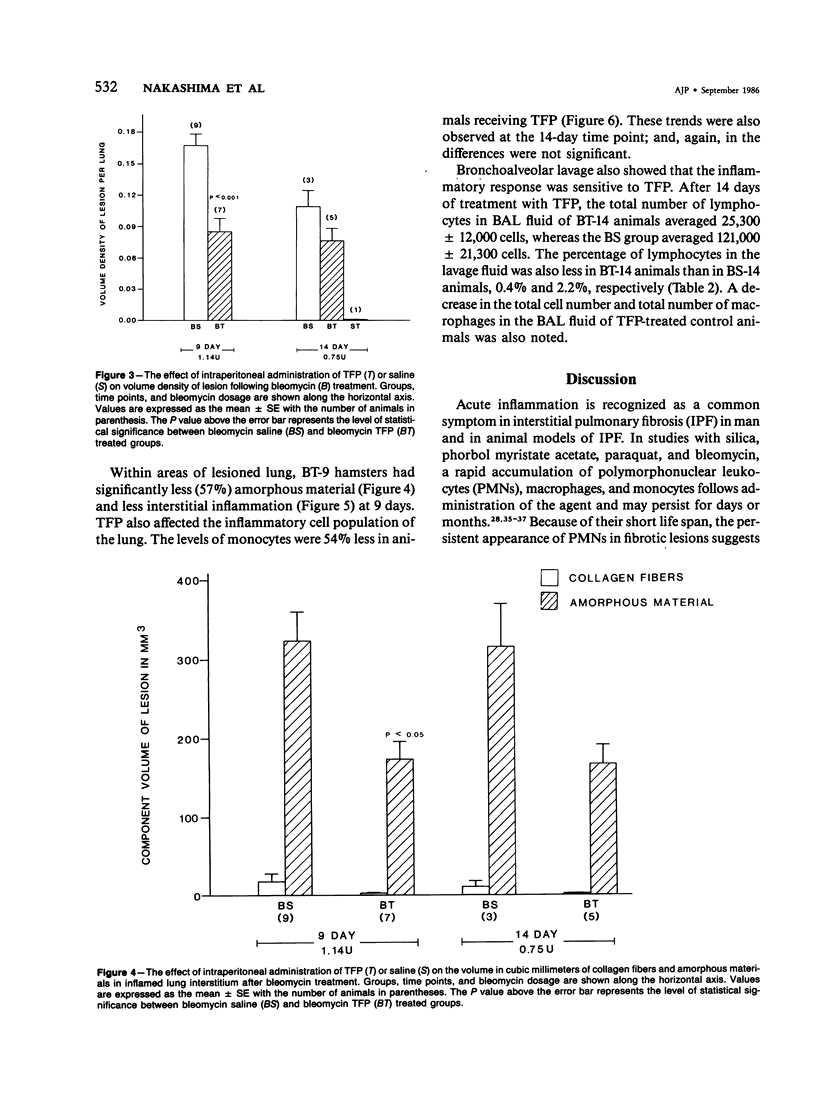
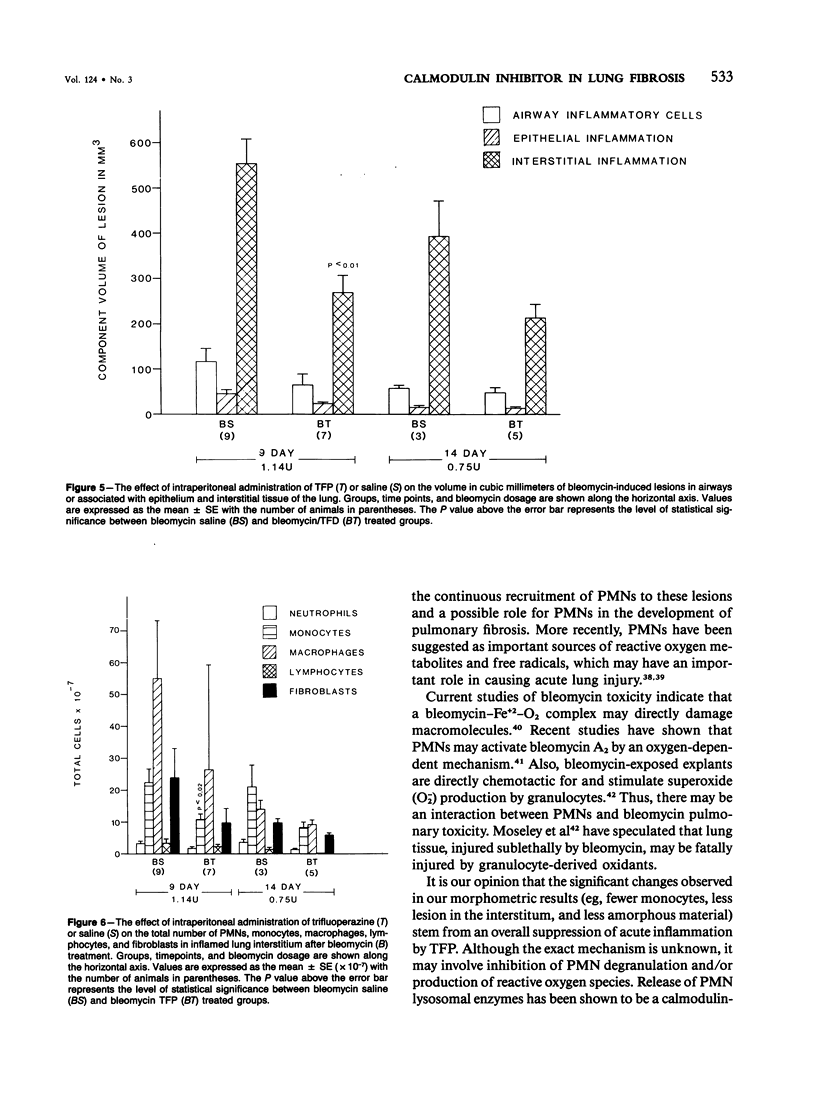
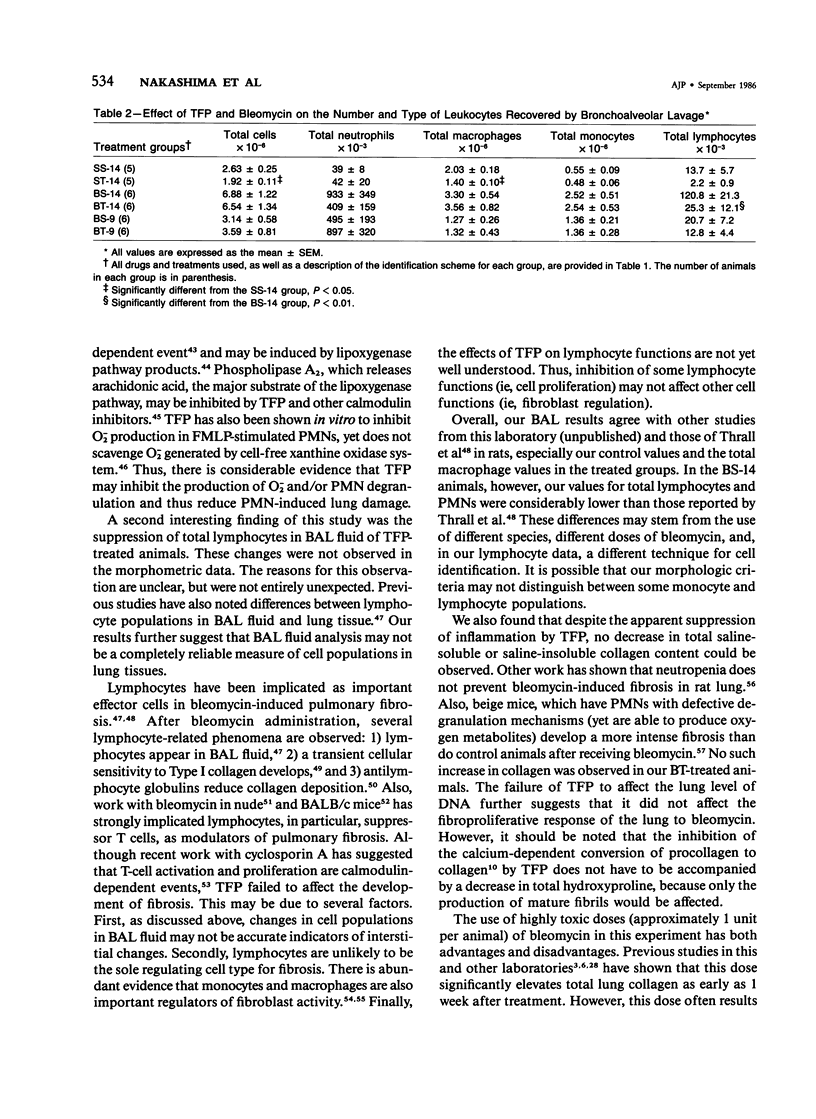
Previous studies have shown that bleomycin-induced pulmonary fibrosis is accompanied by elevated levels of calcium and calmodulin, which are important in the regulation of many biologic processes. The authors have further extended these observations and assessed the effect of a calmodulin inhibitor, trifluoperazine, on bleomycin-induced lung damage with biochemical, morphometric, and bronchoalveolar lavage techniques. The cumulative mortality due to bleomycin was not significantly reduced in animals receiving trifluoperazine. Trifluoperazine had no apparent effect on lung levels of collagen and DNA elevated by bleomycin. However, morphometric studies showed that the volume density of the lesion, the volume of amorphous material and interstitial inflammation, and the number of monocytes within lesions were less in the lungs of bleomycin-treated hamsters receiving trifluoperazine daily. When compared with hamsters treated with bleomycin alone, animals treated with both bleomycin and trifluoperazine had significantly fewer lymphocytes in their bronchoalveolar lavage fluid. The data suggest that trifluoperazine reduced the acute inflammation which accompanies bleomycin pneumotoxicity but did not affect the subsequent development of pulmonary fibrosis. It has been postulated that the observed antiinflammatory action of trifluoperazine may be due to inhibition of calmodulin-dependent leukocyte functions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R. H., Carter S. K., Agre K. A clinical review of bleomycin--a new antineoplastic agent. Cancer. 1973 Apr;31(4):903–914. doi: 10.1002/1097-0142(197304)31:4<903::aid-cncr2820310422>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The role of cell injury and the continuing inflammatory response in the generation of silicotic pulmonary fibrosis. J Pathol. 1984 Nov;144(3):149–161. doi: 10.1002/path.1711440302. [DOI] [PubMed] [Google Scholar]
- Breyer U., Schmalzing G. Metabolism and disposition of trifluoperazine in the rat. I. A thin-layer chromatographic method for the measurement of trifluoperazine and its metabolites in rat tissues. Drug Metab Dispos. 1977 Mar-Apr;5(2):97–103. [PubMed] [Google Scholar]
- Bridges K., Levenson R., Housman D., Cantley L. Calcium regulates the commitment of murine erythroleukemia cells to terminal erythroid differentiation. J Cell Biol. 1981 Aug;90(2):542–544. doi: 10.1083/jcb.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Means A. R. Potentiation of bleomycin lethality by anticalmodulin drugs: a role for calmodulin in DNA repair. Science. 1984 Jun 22;224(4655):1346–1348. doi: 10.1126/science.6203171. [DOI] [PubMed] [Google Scholar]
- Chandler D. B., Hyde D. M., Giri S. N. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol. 1983 Aug;112(2):170–177. [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani P. M., Robb A., Hess A. D. Cyclosporin A binding to calmodulin: a possible site of action on T lymphocytes. Science. 1985 Apr 19;228(4697):337–339. doi: 10.1126/science.3885394. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Marsh-Salin J., Ingram P., Pratt P. C. Tolerance and cross-tolerance using NO2 and O2 II. Pulmonary morphology and morphometry. J Appl Physiol Respir Environ Exerc Physiol. 1978 Mar;44(3):370–379. doi: 10.1152/jappl.1978.44.3.370. [DOI] [PubMed] [Google Scholar]
- Cruz-Orive L. M., Weibel E. R. Sampling designs for stereology. J Microsc. 1981 Jun;122(Pt 3):235–257. doi: 10.1111/j.1365-2818.1981.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Rossman M. D., Zurier R. B., Daniele R. P. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985 Jan;131(1):94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Zurier R. B., Schreiber A. D., Leff J. A., Daniele R. P. Monocyte inhibition of lung fibroblast growth: relationship to fibroblast prostaglandin production and density-defined monocyte subpopulations. J Leukoc Biol. 1985 Jan;37(1):15–28. doi: 10.1002/jlb.37.1.15. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Giri S. N., Chen Z. L., Younker W. R., Schiedt M. J. Effects of intratracheal administration of bleomycin on GSH-shuttle enzymes, catalase, lipid peroxidation, and collagen content in the lungs of hamsters. Toxicol Appl Pharmacol. 1983 Oct;71(1):132–141. doi: 10.1016/0041-008x(83)90052-2. [DOI] [PubMed] [Google Scholar]
- Giri S. N., Hollinger M. A., Schiedt M. J. Effects in vitro and in vivo of thioureas on acetylcholine esterase activity of rat tissues. Biochem Pharmacol. 1977 Feb 15;26(4):313–317. doi: 10.1016/0006-2952(77)90183-6. [DOI] [PubMed] [Google Scholar]
- Giri S. N., Misra H. P., Chandler D. B., Chen Z. L. Increases in lung prolyl hydroxylase and superoxide dismutase activities during bleomycin-induced lung fibrosis in hamsters. Exp Mol Pathol. 1983 Dec;39(3):317–326. doi: 10.1016/0014-4800(83)90060-6. [DOI] [PubMed] [Google Scholar]
- Giri S. N., Nakashima J. M., Curry D. L. Effects of intratracheal administration of bleomycin or saline in pair-fed and control-fed hamsters on daily food intake and on plasma levels of glucose, cortisol, and insulin, and lung levels of calmodulin, calcium, and collagen. Exp Mol Pathol. 1985 Apr;42(2):206–219. doi: 10.1016/0014-4800(85)90028-0. [DOI] [PubMed] [Google Scholar]
- Giri S. N., Schwartz L. W., Hollinger M. A., Freywald M. E., Schiedt M. J., Zuckerman J. E. Biochemical and structural alterations of hamster lungs in response to intratracheal administration of bleomycin. Exp Mol Pathol. 1980 Aug;33(1):1–14. doi: 10.1016/0014-4800(80)90002-7. [DOI] [PubMed] [Google Scholar]
- Gnegy M. E., Erickson R. P., Markovac J. Increased calmodulin in cultured skin fibroblasts from patients with cystic fibrosis. Biochem Med. 1981 Dec;26(3):294–298. doi: 10.1016/0006-2944(81)90004-1. [DOI] [PubMed] [Google Scholar]
- Gross P., DeTreville T. P., Tolker E. B., Kaschak M., Babyak M. A. The pulmonary macrophage response to irritants. An attempt at quantitation. Arch Environ Health. 1969 Feb;18(2):174–185. doi: 10.1080/00039896.1969.10665390. [DOI] [PubMed] [Google Scholar]
- Hickie R. A., Wei J. W., Blyth L. M., Wong D. Y., Klaassen D. J. Cations and calmodulin in normal and neoplastic cell growth regulation. Can J Biochem Cell Biol. 1983 Aug;61(8):934–941. doi: 10.1139/o83-119. [DOI] [PubMed] [Google Scholar]
- Lazo J. S., Hait W. N., Kennedy K. A., Braun I. D., Meandzija B. Enhanced bleomycin-induced DNA damage and cytotoxicity with calmodulin antagonists. Mol Pharmacol. 1985 Mar;27(3):387–393. [PubMed] [Google Scholar]
- Luthra M. G. Trifluoperazine inhibition of calmodulin-sensitive Ca2+ -ATPase and calmodulin insensitive (Na+ +K+)- and Mg2+ -ATPase activities of human and rat red blood cells. Biochim Biophys Acta. 1982 Nov 8;692(2):271–277. doi: 10.1016/0005-2736(82)90531-4. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Whitfield J. F., Boynton A. L., Rixon R. H. Role of cyclic nucleotides and calcium in the positive control of cell proliferation. Adv Cyclic Nucleotide Res. 1975;5:719–734. [PubMed] [Google Scholar]
- Marone G., Poto S., Columbo M., Giugliano R., Genovese A., Condorelli M. Possible role of calmodulin in the control of lysosomal enzyme release from human polymorphonuclear leukocytes. J Pharmacol Exp Ther. 1984 Dec;231(3):678–684. [PubMed] [Google Scholar]
- Moseley P. L., Shasby D. M., Brady M., Hunninghake G. W. Lung parenchymal injury induced by bleomycin. Am Rev Respir Dis. 1984 Dec;130(6):1082–1086. doi: 10.1164/arrd.1984.130.6.1082. [DOI] [PubMed] [Google Scholar]
- Murugesan N., Ehrenfeld G. M., Hecht S. M. Oxygen transfer from bleomycin-metal complexes. J Biol Chem. 1982 Aug 10;257(15):8600–8603. [PubMed] [Google Scholar]
- Oikarinen A. I., Zaragoza E. J., Ryhänen L., Uitto J. Calcium-dependent conversion of procollagen to collagen and its inhibition by other divalent cations. Biochem Pharmacol. 1984 Feb 15;33(4):695–697. doi: 10.1016/0006-2952(84)90331-9. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Schrier D., McGarry B., Duque R. E. Effect of the beige mutation on bleomycin-induced pulmonary fibrosis in mice. Am Rev Respir Dis. 1983 Apr;127(4):456–459. doi: 10.1164/arrd.1983.127.4.456. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Saltini C., Hance A. J., Ferrans V. J., Basset F., Bitterman P. B., Crystal R. G. Accurate quantification of cells recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1984 Oct;130(4):650–658. doi: 10.1164/arrd.1984.130.4.650. [DOI] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970 Jun;26(1):57–60. [PubMed] [Google Scholar]
- Schoenberger C. I., Rennard S. I., Bitterman P. B., Fukuda Y., Ferrans V. J., Crystal R. G. Paraquat-induced pulmonary fibrosis. Role of the alveolitis in modulating the development of fibrosis. Am Rev Respir Dis. 1984 Jan;129(1):168–173. doi: 10.1164/arrd.1984.129.1.168. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H., McGarry B. M. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis. 1983 May;127(5):614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H. Modulation of bleomycin-induced pulmonary fibrosis in the BALB/c mouse by cyclophosphamide-sensitive T cells. Am J Pathol. 1984 Aug;116(2):270–278. [PMC free article] [PubMed] [Google Scholar]
- Schrier D. J., Phan S. H., Ward P. A. Cellular sensitivity to collagen in bleomycin-treated rats. J Immunol. 1982 Nov;129(5):2156–2159. [PubMed] [Google Scholar]
- Smith R. J., Bowman B. J., Iden S. S. Effects of trifluoperazine on human neutrophil function. Immunology. 1981 Dec;44(4):677–684. [PMC free article] [PubMed] [Google Scholar]
- Snider G. L., Celli B. R., Goldstein R. H., O'Brien J. J., Lucey E. C. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis. 1978 Feb;117(2):289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Hayes J. A., Korthy A. L. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: pathology and stereology. Am Rev Respir Dis. 1978 Jun;117(6):1099–1108. doi: 10.1164/arrd.1978.117.6.1099. [DOI] [PubMed] [Google Scholar]
- Starcher B. C., Kuhn C., Overton J. E. Increased elastin and collagen content in the lungs of hamsters receiving an intratracheal injection of bleomycin. Am Rev Respir Dis. 1978 Feb;117(2):299–305. doi: 10.1164/arrd.1978.117.2.299. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol. 1980 May;124(5):2100–2104. [PubMed] [Google Scholar]
- Stevens F. C. Calmodulin: an introduction. Can J Biochem Cell Biol. 1983 Aug;61(8):906–910. doi: 10.1139/o83-115. [DOI] [PubMed] [Google Scholar]
- Taylor R. G., McCall C. E., Thrall R. S., Woodruff R. D., O'Flaherty J. T. Histopathologic features of phorbol myristate acetate-induced lung injury. Lab Invest. 1985 Jan;52(1):61–70. [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W. A comparison of lymphocyte populations in lung tissue and in bronchoalveolar lavage fluid of rats at various times during the development of bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1984 Feb;129(2):279–283. [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W., D'Amato D. A., Sulavik S. B. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982 Sep;126(3):488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Phan S. H., McCormick J. R., Ward P. A. The development of bleomycin-induced pulmonary fibrosis in neutrophil-depleted and complement-depleted rats. Am J Pathol. 1981 Oct;105(1):76–81. [PMC free article] [PubMed] [Google Scholar]
- Trush M. A. Activation of bleomycin A2 to a DNA-damaging intermediate by phorbol ester-stimulated human polymorphonuclear leukocytes. Toxicol Lett. 1984 Mar;20(3):297–302. doi: 10.1016/0378-4274(84)90163-2. [DOI] [PubMed] [Google Scholar]
- Veigl M. L., Vanaman T. C., Sedwick W. D. Calcium and calmodulin in cell growth and transformation. Biochim Biophys Acta. 1984;738(1-2):21–48. doi: 10.1016/0304-419x(84)90018-0. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Banks W. L., Jr, Wunner W. H. Use of a single tissue extract to determine cellular protein and nucleic acid concentrations and rate of amino acid incorporation. Anal Biochem. 1965 May;11(2):320–326. doi: 10.1016/0003-2697(65)90020-5. [DOI] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- West N. R., Vogel W. H. Absorption, distribution and excretion of trifluoperazine in rats. Arch Int Pharmacodyn Ther. 1975 Jun;215(2):318–335. [PubMed] [Google Scholar]
- Wong P. Y., Lee W. H., Chao P. H., Cheung W. Y. The role of calmodulin in prostaglandin metabolism. Ann N Y Acad Sci. 1980;356:179–189. doi: 10.1111/j.1749-6632.1980.tb29610.x. [DOI] [PubMed] [Google Scholar]
- van de Kerkhof P. C., van Erp P. E. Calmodulin levels are grossly elevated in the psoriatic lesion. Br J Dermatol. 1983 Feb;108(2):217–218. doi: 10.1111/j.1365-2133.1983.tb00066.x. [DOI] [PubMed] [Google Scholar]


