Abstract
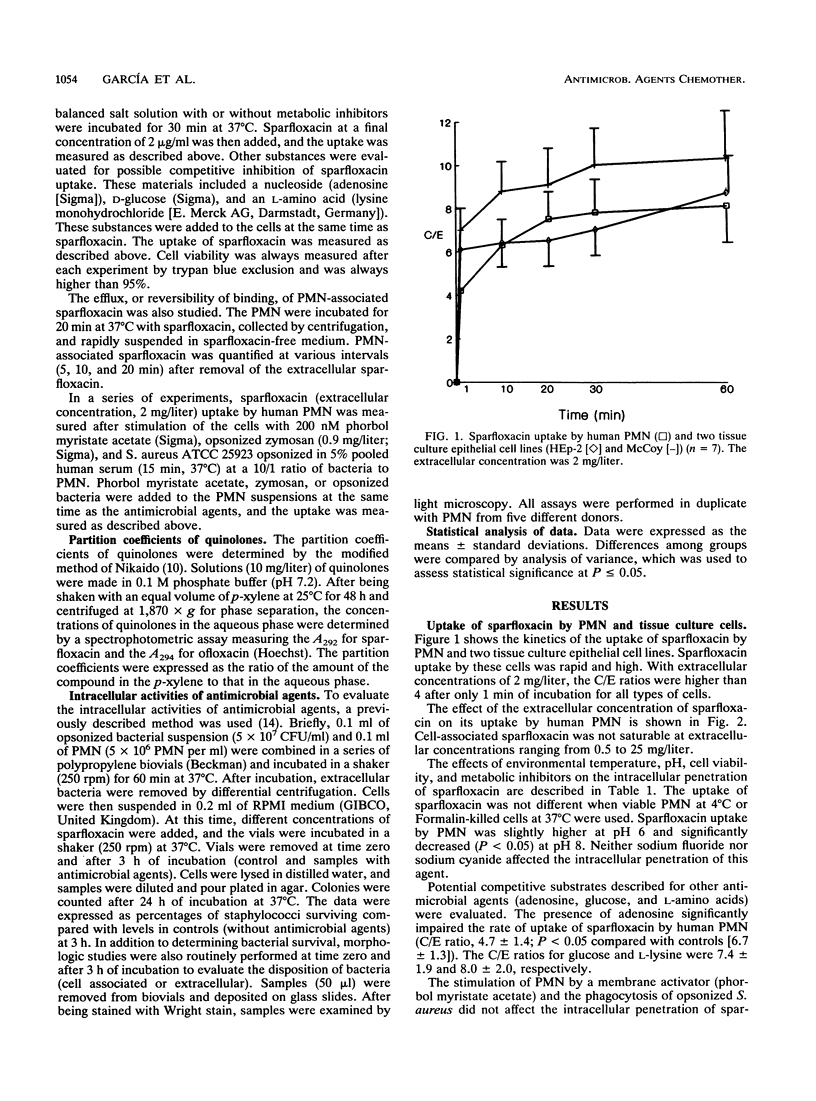
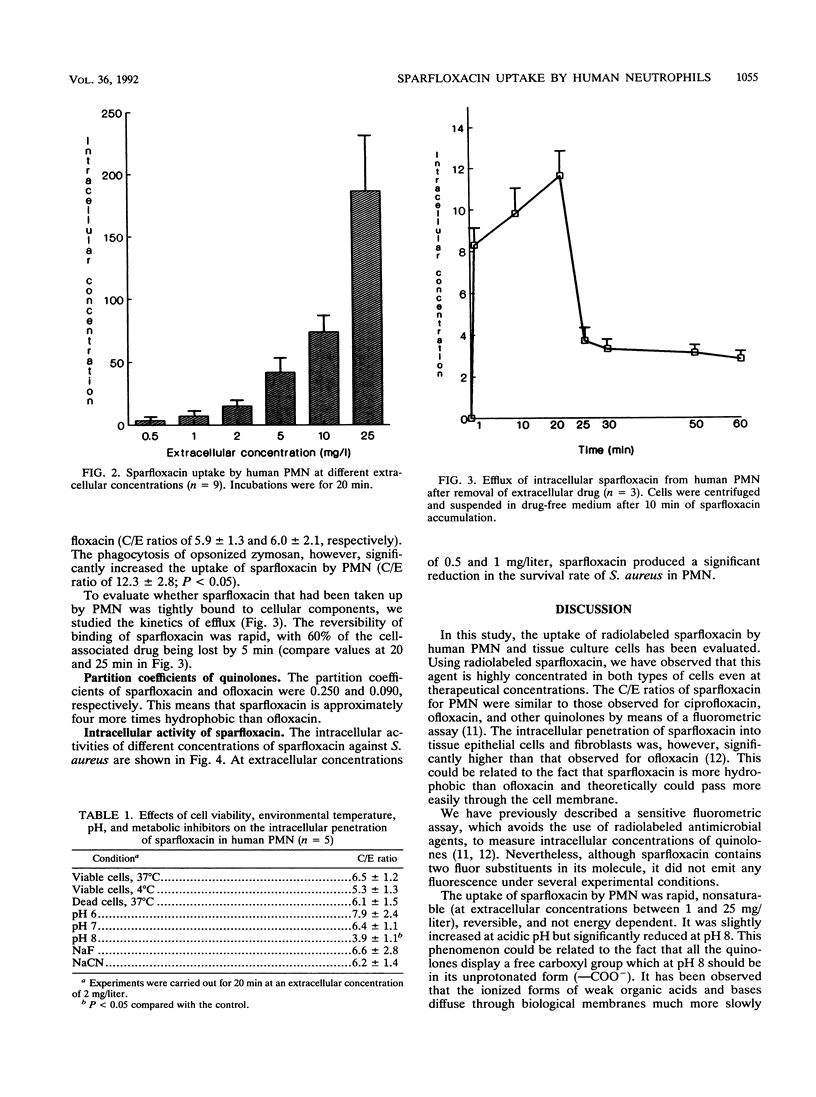
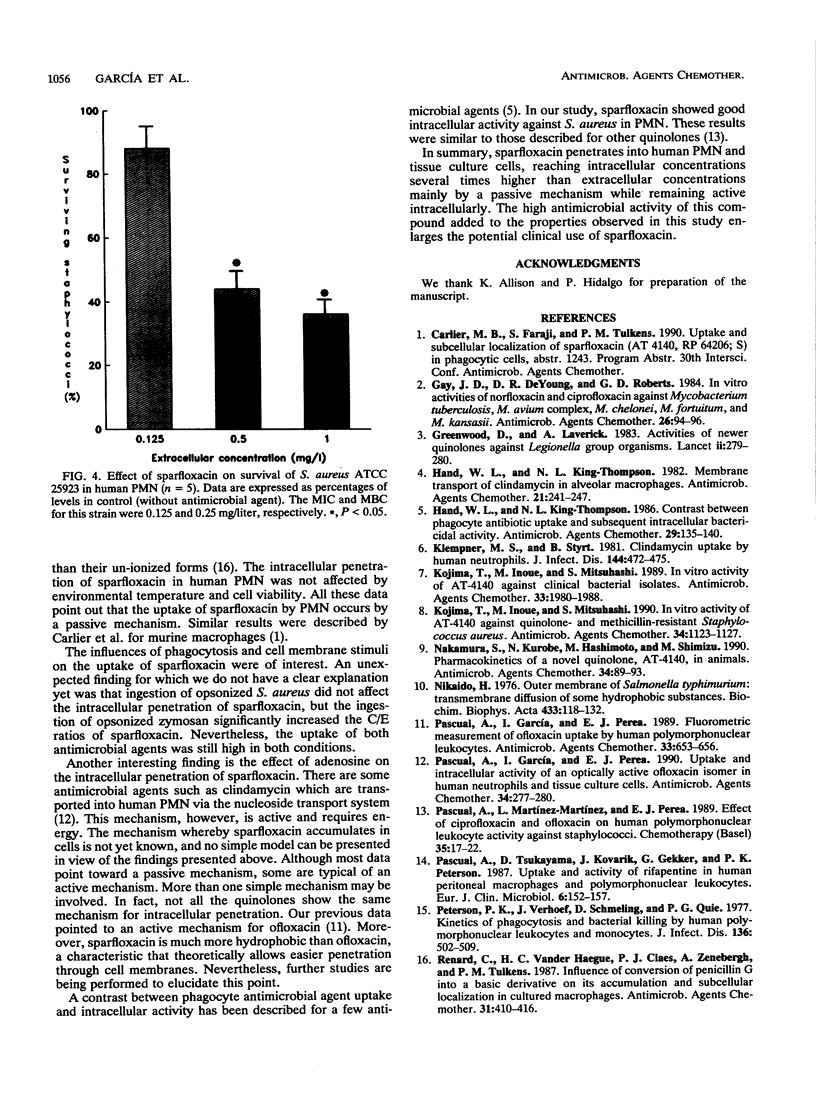
The penetration of sparfloxacin into human neutrophils (PMN) and different tissue culture cells (HEp-2 and McCoy) was evaluated. The cellular to extracellular concentration ratios (C/E) of sparfloxacin were always higher than 4 at extracellular concentrations ranging from 0.5 to 25 mg/liter. The uptake of sparfloxacin by PMN was rapid, nonsaturable, reversible, not energy dependent, and significantly reduced at pH 8. The penetration of this agent into PMN was similar when viable and Formalin-killed cells were used and was not affected by environmental temperature. Ingestion of opsonized zymosan significantly increased the amount of PMN-associated sparfloxacin. Sparfloxacin at a concentration of 0.5 mg induced a significant reduction in the survival of intracellular Staphylococcus aureus. It is concluded that sparfloxacin reaches intracellular concentrations within leukocytic cells much higher than extracellular concentrations, while remaining active intracellularly.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus B. L., Fyfe J. A., Hancock R. E. Mapping and characterization of two mutations to antibiotic supersusceptibility in Pseudomonas aeruginosa. J Gen Microbiol. 1987 Oct;133(10):2905–2914. doi: 10.1099/00221287-133-10-2905. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Heritage J., Hawkey P. M. An ultra-rapid method for the study of antibiotic resistance plasmids. J Antimicrob Chemother. 1986 Sep;18(3):421–424. doi: 10.1093/jac/18.3.421. [DOI] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1983 Nov;24(5):754–763. doi: 10.1128/aac.24.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay J. D., DeYoung D. R., Roberts G. D. In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrob Agents Chemother. 1984 Jul;26(1):94–96. doi: 10.1128/aac.26.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., Laverick A. Activities of newer quinolones against Legionella group organisms. Lancet. 1983 Jul 30;2(8344):279–280. doi: 10.1016/s0140-6736(83)90257-x. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Billot-Klein D., Williamson R., Goldstein F. W., Mounier J., Acar J. F., Collatz E. Mutation of Salmonella paratyphi A conferring cross-resistance to several groups of antibiotics by decreased permeability and loss of invasiveness. Antimicrob Agents Chemother. 1988 Feb;32(2):195–201. doi: 10.1128/aac.32.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Siehnel R., Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990 Jul;4(7):1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Contrasts between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986 Jan;29(1):135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Membrane transport of clindamycin in alveolar macrophages. Antimicrob Agents Chemother. 1982 Feb;21(2):241–247. doi: 10.1128/aac.21.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M. Mechanism of ciprofloxacin resistance in Pseudomonas aeruginosa. J Infect Dis. 1988 Sep;158(3):537–541. doi: 10.1093/infdis/158.3.537. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1981 Nov;144(5):472–479. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against clinical bacterial isolates. Antimicrob Agents Chemother. 1989 Nov;33(11):1980–1988. doi: 10.1128/aac.33.11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against quinolone- and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Jun;34(6):1123–1127. doi: 10.1128/aac.34.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis N. J., Tzouvelekis L. S., Makris A., Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Masecar B. L., Celesk R. A., Robillard N. J. Analysis of acquired ciprofloxacin resistance in a clinical strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Feb;34(2):281–286. doi: 10.1128/aac.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Michea-Hamzehpour M., Lucain C., Pechere J. C. Resistance to pefloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Mar;35(3):512–518. doi: 10.1128/aac.35.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Auckenthaler R., Regamey P., Pechère J. C. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob Agents Chemother. 1987 Nov;31(11):1803–1808. doi: 10.1128/aac.31.11.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. G., Piddock L. J. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1991 Nov;28(5):639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Characterization of two surface-localized antigenic sites on porin protein F of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):381–386. doi: 10.1139/m85-073. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kurobe N., Ohue T., Hashimoto M., Shimizu M. Pharmacokinetics of a novel quinolone, AT-4140, in animals. Antimicrob Agents Chemother. 1990 Jan;34(1):89–93. doi: 10.1128/aac.34.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Harayama S. Identification and characterization of porins in Pseudomonas aeruginosa. J Biol Chem. 1991 Jan 15;266(2):770–779. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Pascual A., Garcia I., Perea E. J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1989 May;33(5):653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A., Garcia I., Perea E. J. Uptake and intracellular activity of an optically active ofloxacin isomer in human neutrophils and tissue culture cells. Antimicrob Agents Chemother. 1990 Feb;34(2):277–280. doi: 10.1128/aac.34.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A., Martínez-Martínez L., Perea E. J. Effect of ciprofloxacin and ofloxacin on human polymorphonuclear leukocyte activity against staphylococci. Chemotherapy. 1989;35(1):17–22. doi: 10.1159/000238631. [DOI] [PubMed] [Google Scholar]
- Pascual A., Tsukayama D., Kovarik J., Gekker G., Peterson P. Uptake and activity of rifapentine in human peritoneal macrophages and polymorphonuclear leukocytes. Eur J Clin Microbiol. 1987 Apr;6(2):152–157. doi: 10.1007/BF02018197. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Traynor E. A., Wise R. A comparison of the mechanisms of decreased susceptibility of aztreonam-resistant and ceftazidime-resistant Enterobacteriaceae. J Antimicrob Chemother. 1990 Dec;26(6):749–762. doi: 10.1093/jac/26.6.749. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wijnands W. J., Wise R. Quinolone/ureidopenicillin cross-resistance. Lancet. 1987 Oct 17;2(8564):907–907. doi: 10.1016/s0140-6736(87)91387-0. [DOI] [PubMed] [Google Scholar]
- Reimmann C., Haas D. IS21 insertion in the trfA replication control gene of chromosomally integrated plasmid RP1: a property of stable Pseudomonas aeruginosa Hfr strains. Mol Gen Genet. 1986 Jun;203(3):511–519. doi: 10.1007/BF00422078. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard C., Vanderhaeghe H. J., Claes P. J., Zenebergh A., Tulkens P. M. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob Agents Chemother. 1987 Mar;31(3):410–416. doi: 10.1128/aac.31.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible beta-lactamases. Antimicrob Agents Chemother. 1979 Jun;15(6):792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wijnands W. J., van Griethuysen A. J., Vree T. B., Van Klingeren B., van Herwaarden C. L. Enoxacin in lower respiratory tract infections. J Antimicrob Chemother. 1986 Dec;18(6):719–727. doi: 10.1093/jac/18.6.719. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Danks G. In-vitro activity of enoxacin (CL-919), a new quinoline derivative, compared with that of other antimicrobial agents. J Antimicrob Chemother. 1984 Mar;13(3):237–244. doi: 10.1093/jac/13.3.237. [DOI] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989 Jun;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jun;34(6):1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E., Nakae T. Identification of porins in the outer membrane of Pseudomonas aeruginosa that form small diffusion pores. J Biol Chem. 1989 Apr 15;264(11):6297–6301. [PubMed] [Google Scholar]


