Abstract
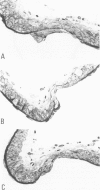
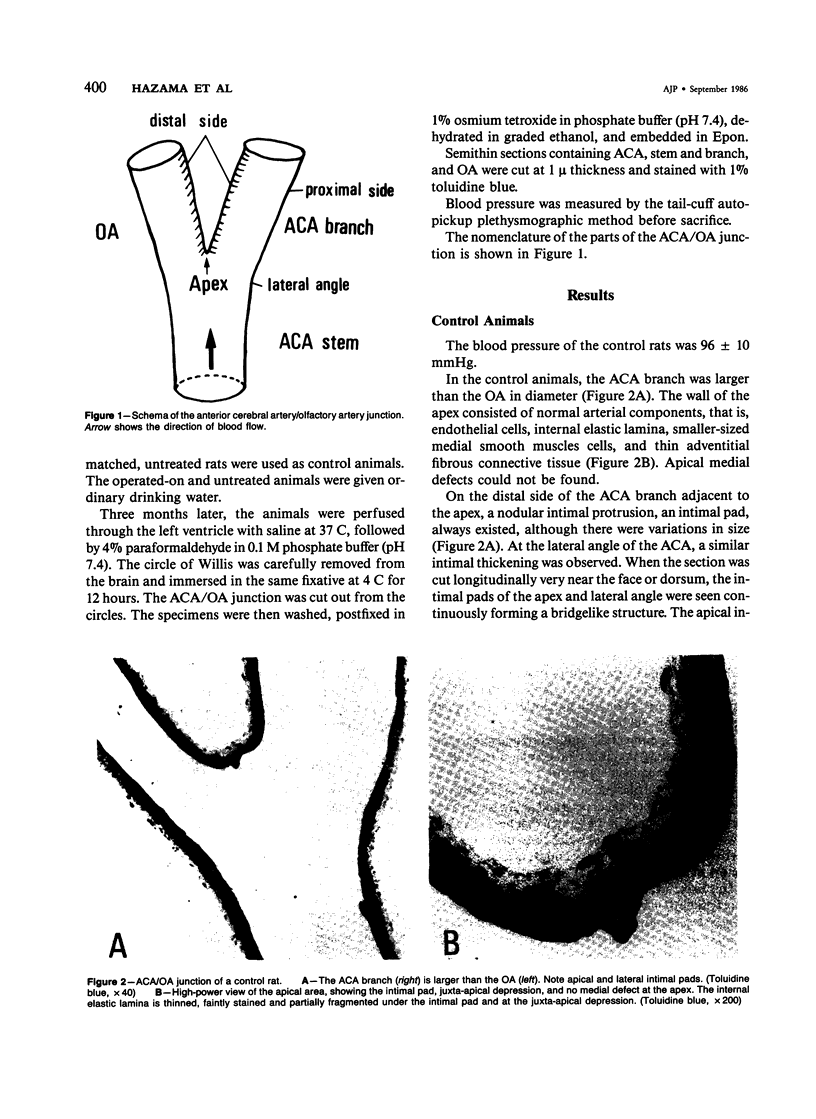
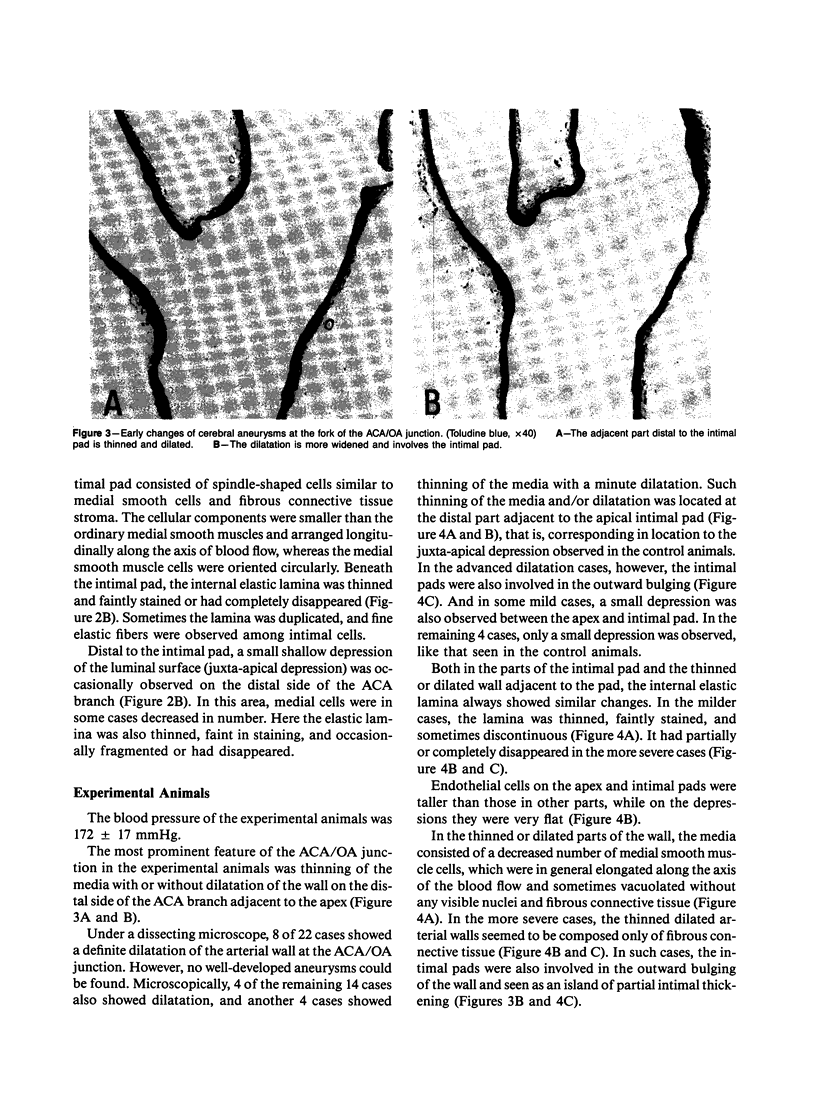
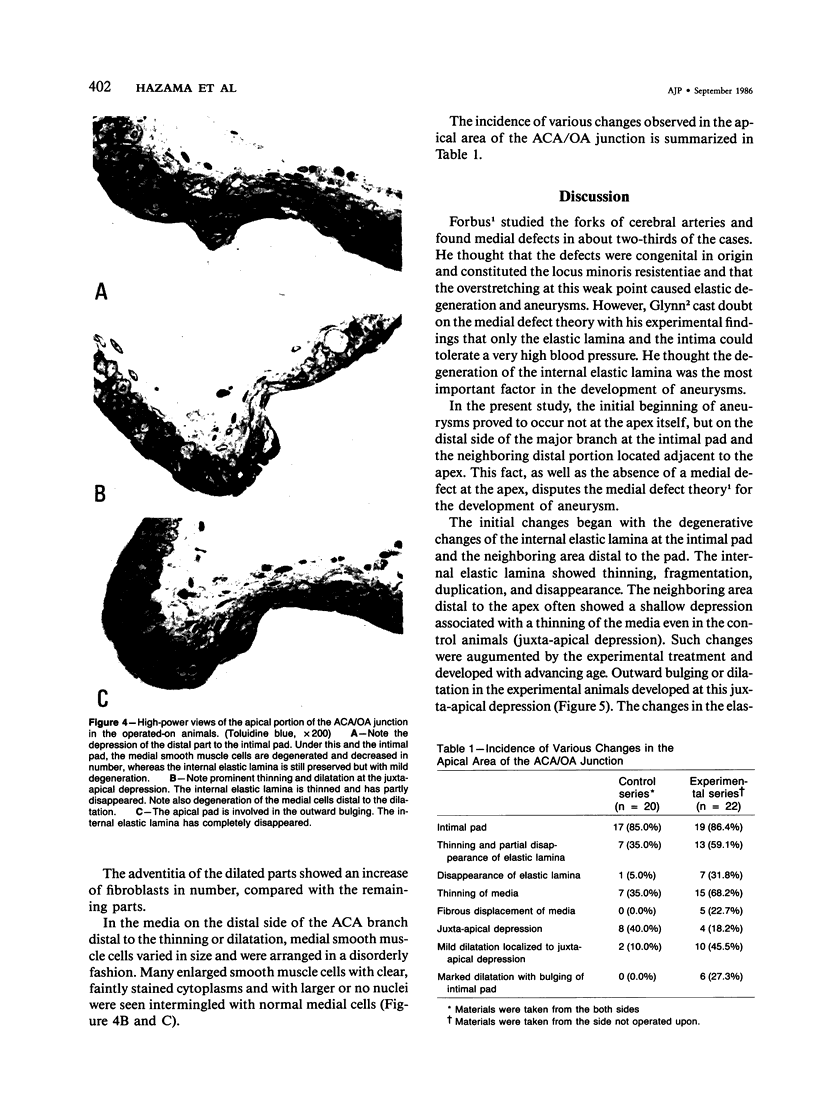
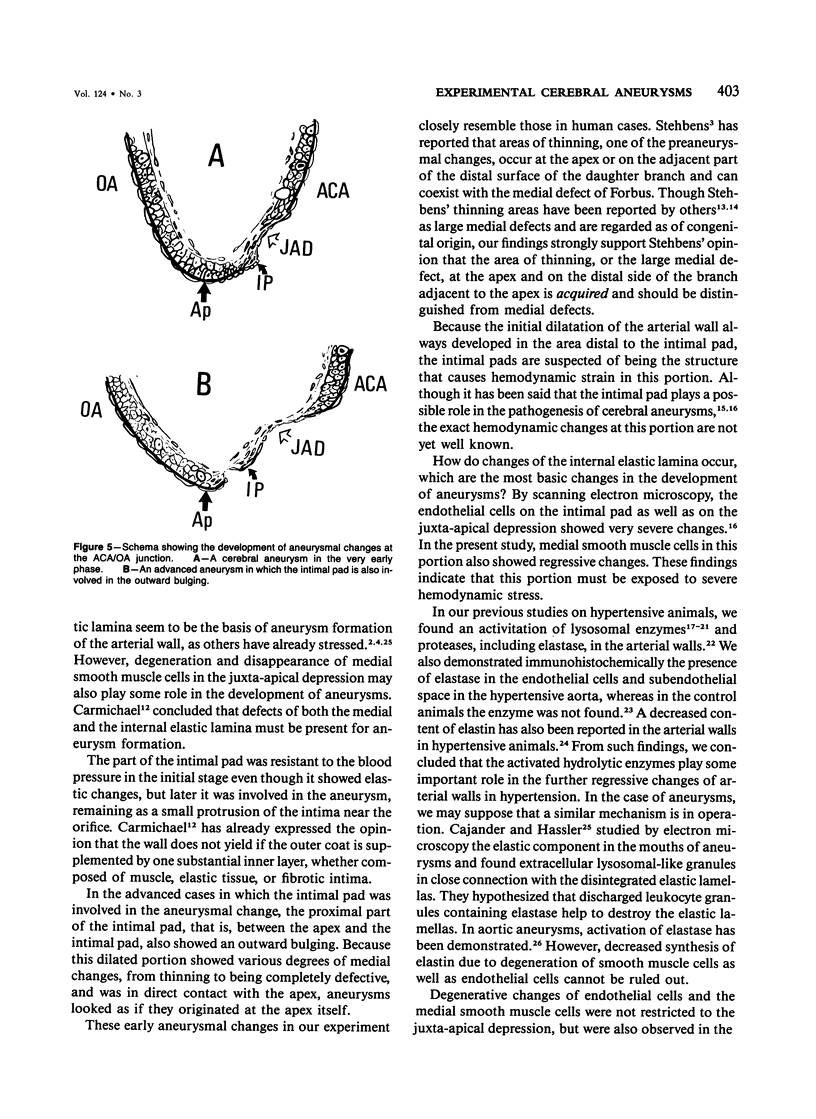
The changes of the anterior cerebral artery/olfactory artery junction, one of the favorite sites of aneurysm formation, in rats treated with unilateral ligation of the common carotid artery and renal hypertension were investigated by light microscopy. The initial changes of aneurysm occurred not at the apex itself, but on the distal side of the major branch adjacent to the apex, at the intimal pad and the neighboring distal portion. Here the internal elastic lamina showed various degenerative changes and disappearance. The neighboring distal portion adjacent to the intimal pad showed a shallow depression associated with a thinning of the media due to a decrease of medial smooth muscle cells in number even in some control animals. Such degenerative changes of the internal elastic lamina and medial smooth muscle cells caused by hemodynamic stress due to branching structure, including intimal pads, augmented by the experimental treatment, are supposed to be the basis for aneurysm formation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnoli A. L. Vascular anomalies and subarachnoid haemorrhage associated with persisting embryonic vessels. Acta Neurochir (Wien) 1982;60(3-4):183–199. doi: 10.1007/BF01406306. [DOI] [PubMed] [Google Scholar]
- Busuttil R. W., Rinderbriecht H., Flesher A., Carmack C. Elastase activity: the role of elastase in aortic aneurysm formation. J Surg Res. 1982 Mar;32(3):214–217. doi: 10.1016/0022-4804(82)90093-2. [DOI] [PubMed] [Google Scholar]
- CARMICHAEL R. The pathogenesis of noninflammatory cerebral aneurysms. J Pathol Bacteriol. 1950 Jan;62(1):1–19. doi: 10.1002/path.1700620102. [DOI] [PubMed] [Google Scholar]
- Cajander S., Hassler O. Enzymatic destruction of the elastic lamella at the mouth of cerebral berry aneurysm? An ultrastructural study with special regard to the elastic tissue. Acta Neurol Scand. 1976 Mar;53(3):171–181. doi: 10.1111/j.1600-0404.1976.tb04335.x. [DOI] [PubMed] [Google Scholar]
- HASSLER O. Morphological studies on the large cerebral arteries, with reference to the aetiology of subarachnoid haemorrhage. Acta Psychiatr Scand Suppl. 1961;154:1–145. [PubMed] [Google Scholar]
- Handa H., Hashimoto N., Nagata I., Hazama F. Saccular cerebral aneurysms in rats: a newly developed animal model of the disease. Stroke. 1983 Nov-Dec;14(6):857–866. doi: 10.1161/01.str.14.6.857. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Handa H., Hazama F. Experimentally induced cerebral aneurysms in rats. Surg Neurol. 1978 Jul;10(1):3–8. [PubMed] [Google Scholar]
- Hashimoto N., Handa H., Nagata I., Hazama F. Animal model of cerebral aneurysms: pathology and pathogenesis of induced cerebral aneurysms in rats. Neurol Res. 1984 Mar-Jun;6(1-2):33–40. doi: 10.1080/01616412.1984.11739661. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Handa H., Nagata I., Hazama F. Saccular cerebral aneurysms in rats. Am J Pathol. 1983 Mar;110(3):397–399. [PMC free article] [PubMed] [Google Scholar]
- STEHBENS W. E. Histopathology of cerebral aneurysms. Arch Neurol. 1963 Mar;8:272–285. doi: 10.1001/archneur.1963.00460030056005. [DOI] [PubMed] [Google Scholar]
- Sasahara M., Hazama F., Yamada E., Kataoka H., Amano S., Kayembe K. Semiquantitative histochemical investigation of lysosomal enzyme activities in the aortic endothelial cells of rats with renal hypertension. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(3):267–274. doi: 10.1007/BF02890176. [DOI] [PubMed] [Google Scholar]
- Sekhar L. N., Heros R. C. Origin, growth, and rupture of saccular aneurysms: a review. Neurosurgery. 1981 Feb;8(2):248–260. doi: 10.1227/00006123-198102000-00020. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Yamada E., Hazama F., Nomura T. Acid phosphatase activity in the aorta of spontaneously hypertensive rats and the effect of various antihypertensive drugs. Atherosclerosis. 1981 Oct;40(2):167–174. doi: 10.1016/0021-9150(81)90035-6. [DOI] [PubMed] [Google Scholar]
- Yamada E., Hazama F., Amano S., Hanakita J. Acid phosphatase activity of the brain in SHR-- the first report of enzyme histochemical studies of spontaneously hypertensive rat brain. Jpn Circ J. 1979 Apr;43(4):285–292. doi: 10.1253/jcj.43.285. [DOI] [PubMed] [Google Scholar]
- Yamada E., Hazama F., Amano S., Hanakita J. Cytochemical investigation on acid phosphatase activity in cerebral arteries in spontaneously hypertensive rats. Jpn Circ J. 1980 Jun;44(6):467–475. doi: 10.1253/jcj.44.467. [DOI] [PubMed] [Google Scholar]
- Yamada E., Hazama F., Amano S., Sasahara M., Kataoka H. Elastase, collagenase, and cathepsin D activities in the aortas of spontaneously hypertensive and renal hypertensive rats. Exp Mol Pathol. 1986 Apr;44(2):147–156. doi: 10.1016/0014-4800(86)90065-1. [DOI] [PubMed] [Google Scholar]
- Yamada E., Hazama F., Amano S., Sasahara M., Kataoka H., Kayembe K. Semiquantitative histochemical investigation of lysosomal enzyme activities in the aortic endothelial cells of spontaneously hypertensive rats. Atherosclerosis. 1983 Apr;47(1):19–26. doi: 10.1016/0021-9150(83)90067-9. [DOI] [PubMed] [Google Scholar]
- Yamada E., Hazama F., Kataoka H., Amano S., Sasahara M., Kayembe K., Katayama K. Elastase-like enzyme in the aorta of spontaneously hypertensive rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(2):241–245. doi: 10.1007/BF02890173. [DOI] [PubMed] [Google Scholar]