Abstract
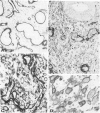
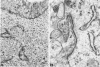
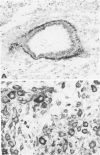
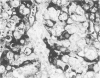
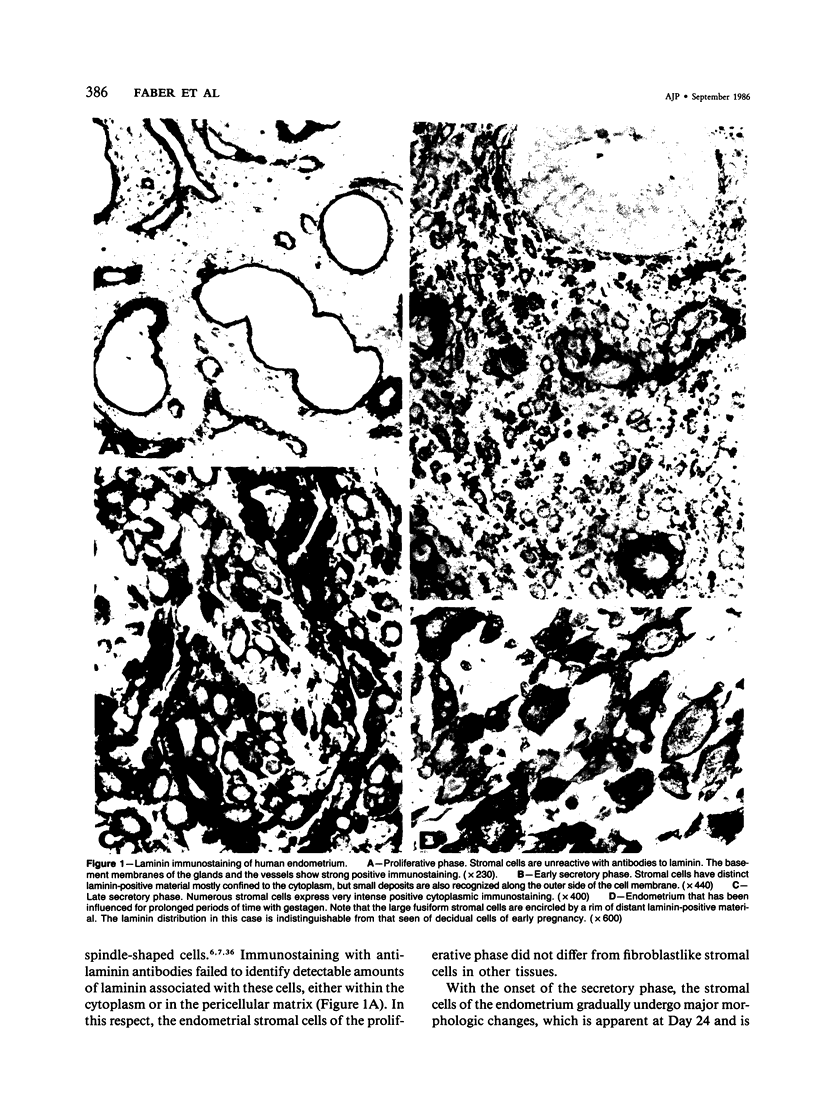
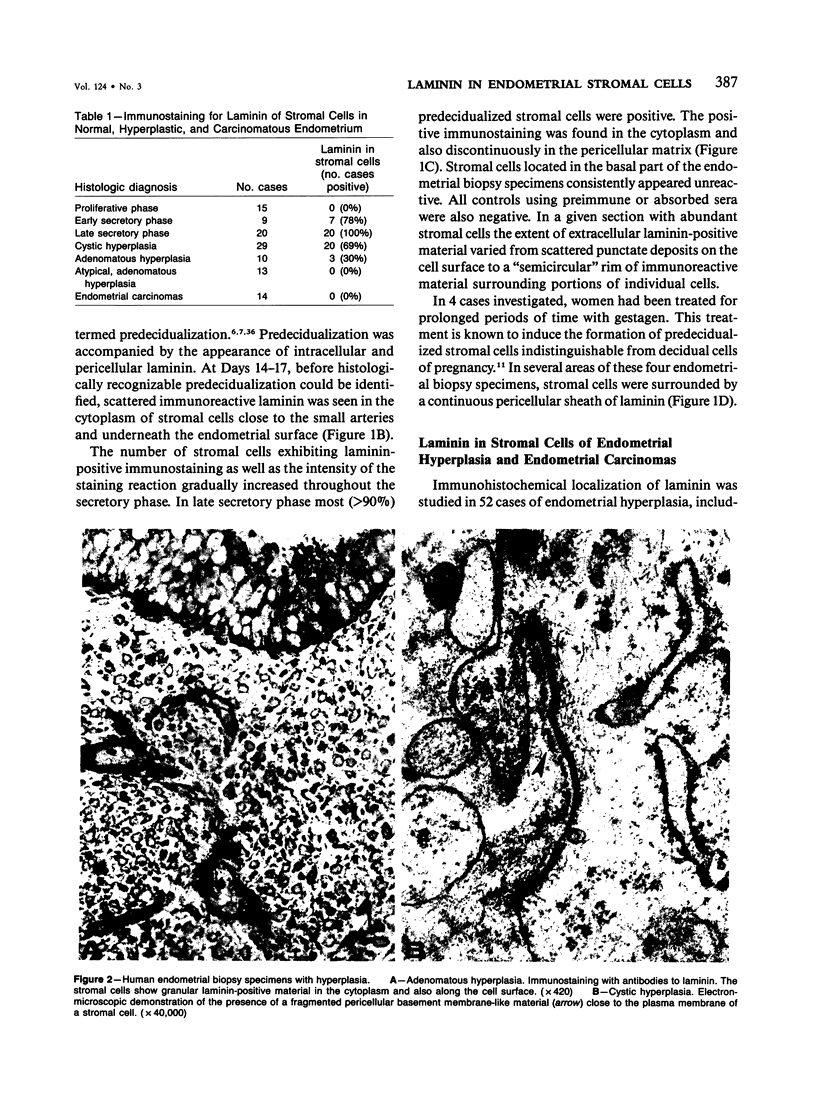
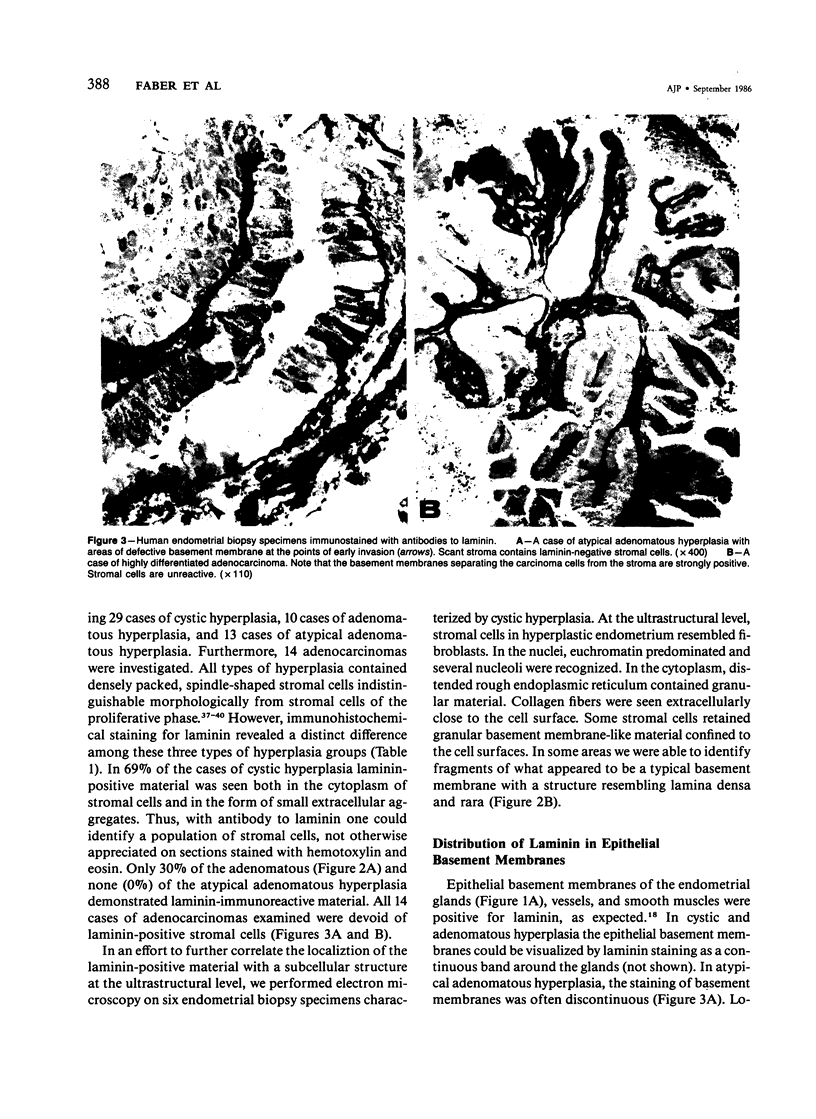
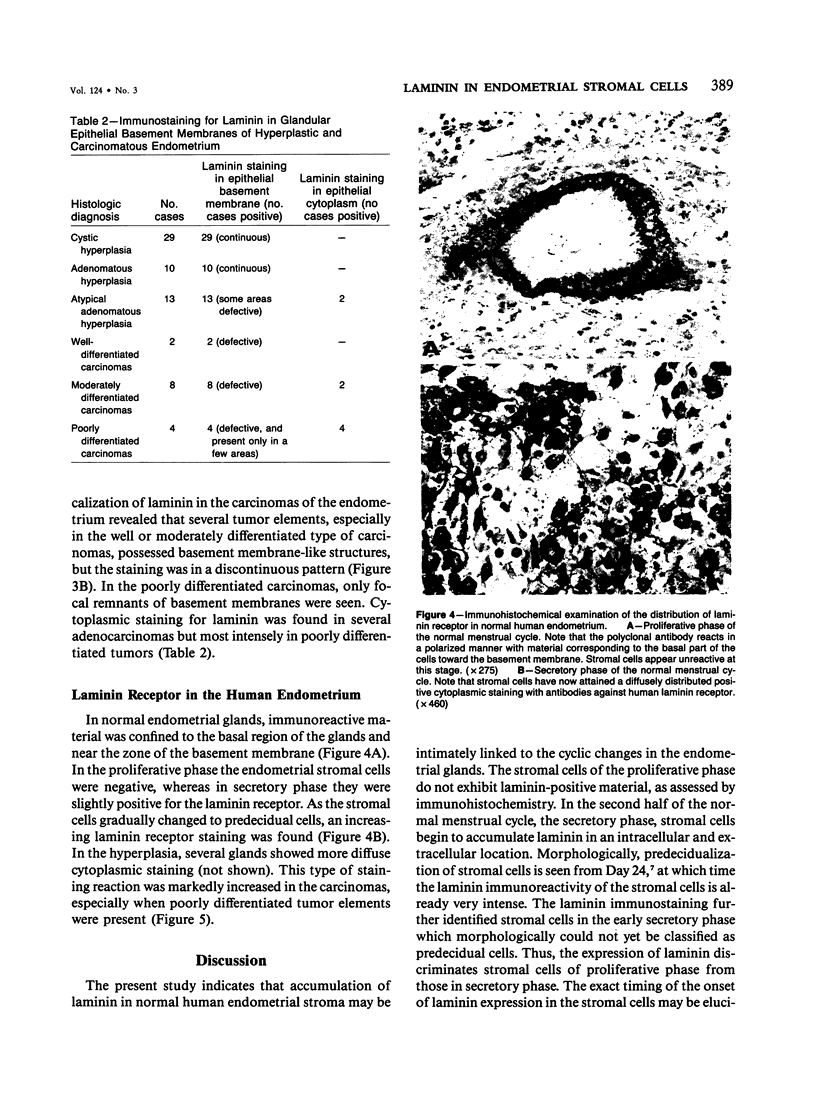
The cyclic changes in the presence of the basement membrane glycoprotein laminin in endometrial stromal cells was studied by immunohistochemistry. The interstitial matrix around the stromal cells of the proliferative phase of the normal menstrual cycle was unreactive with antibodies to laminin. However, commencing with the secretory phase, stromal cells accumulated distinct cytoplasmic and pericellular laminin-immunoreactive material. The maximal amount of stromal cell-associated laminin was observed in predecidual cells of the late secretory phase. Thus, laminin immunostaining discriminates stromal cells of the proliferative phase (being "negative") from those in the secretory phase (being "positive"). Sixty-six cases of endometrial hyperplasia and adenocarcinomas were also stained with antibodies to laminin. Sixty-nine percent of biopsies of cystic hyperplasia and 30% of adenomatous hyperplasia contained laminin-positive stromal cells. Ultrastructural examination of stromal cells in cystic hyperplasia revealed the presence of pericellular basement membrane-like material, focally arranged into typical lamina rara and lamina densa. In contrast, stromal cells in the atypical adenomatous hyperplasia and adenocarcinomas did not react with antibody to laminin. The expression of laminin receptor in the stromal cells codistributed with laminin. Basement membranes of the surface epithelium, the glandular epithelium, and the vessels stained strongly with antibodies to laminin. In preneoplastic and neoplastic tissues, laminin immunostaining revealed discontinuous and defective basement membranes. In poorly differentiated carcinomas only sparse amounts of laminin-positive basement membrane were observed; these tumors, in contrast, exhibited cytoplasmic laminin and also significant immunoreaction with antibodies to laminin receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Albrechtsen R., Wewer U. M., Liotta L. A. Basement membranes in human cancer. Pathol Annu. 1986;21(Pt 2):251–276. [PubMed] [Google Scholar]
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Burns J., Dixon A. J., Woods J. C. Immunoperoxidase localisation of fibronectin in glomeruli of formalin fixed paraffin processed renal tissue. Histochemistry. 1980;67(1):73–78. doi: 10.1007/BF00490089. [DOI] [PubMed] [Google Scholar]
- Charpin C., Kopp F., Pourreau-Schneider N., Lissitzky J. C., Lavaut M. N., Martin P. M., Toga M. Laminin distribution in human decidua and immature placenta. An immunoelectron microscopic study (avidin-biotin-peroxidase complex method). Am J Obstet Gynecol. 1985 Mar 15;151(6):822–826. doi: 10.1016/0002-9378(85)90529-0. [DOI] [PubMed] [Google Scholar]
- Damjanov I. Vesalius and Hunter were right: decidua is a membrane! Lab Invest. 1985 Dec;53(6):597–598. [PubMed] [Google Scholar]
- Engvall E., Krusius T., Wewer U., Ruoslahti E. Laminin from rat yolk sac tumor: isolation, partial characterization, and comparison with mouse laminin. Arch Biochem Biophys. 1983 Apr 15;222(2):649–656. doi: 10.1016/0003-9861(83)90562-3. [DOI] [PubMed] [Google Scholar]
- Fenoglio C. M., Crum C. P., Ferenczy A. Endometrial hyperplasia and carcinoma. Are ultrastructural, biochemical and immunocytochemical studies useful in distinguishing between them? Pathol Res Pract. 1982 Aug;174(3):257–284. doi: 10.1016/S0344-0338(82)80070-8. [DOI] [PubMed] [Google Scholar]
- Fox H., Buckley C. H. The endometrial hyperplasias and their relationship to endometrial neoplasia. Histopathology. 1982 Sep;6(5):493–510. doi: 10.1111/j.1365-2559.1982.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Haglund C., Roberts P. J., Nordling S., Ekblom P. Expression of laminin in pancreatic neoplasms and in chronic pancreatitis. Am J Surg Pathol. 1984 Sep;8(9):669–676. doi: 10.1097/00000478-198409000-00006. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Kurman R. J., Kaminski P. F., Norris H. J. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985 Jul 15;56(2):403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kurman R. J., Norris H. J. Evaluation of criteria for distinguishing atypical endometrial hyperplasia from well-differentiated carcinoma. Cancer. 1982 Jun 15;49(12):2547–2559. doi: 10.1002/1097-0142(19820615)49:12<2547::aid-cncr2820491224>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Martin P. M., Rolland P. H., Gammerre M., Serment H., Toga M. Estradiol and progesterone receptors in normal and neoplastic endometrium: correlations between receptors, histopathological examinations and clinical responses under progestin therapy. Int J Cancer. 1979 Mar 15;23(3):321–329. doi: 10.1002/ijc.2910230309. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Maruffo C. A., Casavilla F., van Nynatten B., Perez V. Modifications of the human endometrial fine structure induced by low-dose progestogen therapy. Fertil Steril. 1974 Sep;25(9):778–787. doi: 10.1016/s0015-0282(16)40634-5. [DOI] [PubMed] [Google Scholar]
- Maslar I. A., Riddick D. H. Prolactin production by human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979 Nov 15;135(6):751–754. doi: 10.1016/0002-9378(79)90386-7. [DOI] [PubMed] [Google Scholar]
- McArdle J. P., Müller H. K., Roff B. T., Murphy W. H. Basal lamina redevelopment in tumours metastatic to brain:an immunoperoxidase study using an antibody to type-IV collagen. Int J Cancer. 1984 Nov 15;34(5):633–638. doi: 10.1002/ijc.2910340508. [DOI] [PubMed] [Google Scholar]
- More I. A., Armstrong E. M., Carty M., McSeveney D. Cyclical changes in the ultrastructure of the normal human endometrial stromal cell. J Obstet Gynaecol Br Commonw. 1974 May;81(5):337–347. doi: 10.1111/j.1471-0528.1974.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Press M. F., Nousek-Goebl N., King W. J., Herbst A. L., Greene G. L. Immunohistochemical assessment of estrogen receptor distribution in the human endometrium throughout the menstrual cycle. Lab Invest. 1984 Nov;51(5):495–503. [PubMed] [Google Scholar]
- Riddick D. H., Daly D. C., Walters C. A. The uterus as an endocrine compartment. Clin Perinatol. 1983 Oct;10(3):627–639. [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C., Chung A. E., Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984 Mar;98(3):971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. R., Scully R. E. Precancerous lesions of the endometrium. Hum Pathol. 1977 Sep;8(5):503–512. doi: 10.1016/s0046-8177(77)80111-1. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Faber M., Liotta L. A., Albrechtsen R. Immunochemical and ultrastructural assessment of the nature of the pericellular basement membrane of human decidual cells. Lab Invest. 1985 Dec;53(6):624–633. [PubMed] [Google Scholar]
- Wewer U., Albrechtsen R., Manthorpe M., Varon S., Engvall E., Ruoslahti E. Human laminin isolated in a nearly intact, biologically active form from placenta by limited proteolysis. J Biol Chem. 1983 Oct 25;258(20):12654–12660. [PubMed] [Google Scholar]
- Wewer U. Characterization of a rat yolk sac carcinoma cell line. Dev Biol. 1982 Oct;93(2):416–421. doi: 10.1016/0012-1606(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Wienke E. C., Jr, Cavazos F., Hall D. G., Lucas F. V. Ultrastructure of the human endometrial stroma cell during the menstrual cycle. Am J Obstet Gynecol. 1968 Sep 1;102(1):65–77. doi: 10.1016/0002-9378(68)90434-1. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Wan Y. J., Chung A. E., Damjanov I. Immunohistochemical localization of entactin and laminin in mouse embryos and fetuses. Dev Biol. 1983 Dec;100(2):496–505. doi: 10.1016/0012-1606(83)90242-7. [DOI] [PubMed] [Google Scholar]