INTRODUCTION
Cartilage tumors present in different shapes, sizes, and grades.20 The vast majority are benign lesions and often require no surgical treatment. The orthopaedic surgeon treating patients with cartilage lesions should be familiar with the subtypes, histological features, and degrees of atypia/grades. Enchondromas are commonly found incidental lesions within the intramedullary canal, and rarely require surgical intervention unless they are clinically symptomatic. Patients with Ollier's disease or Maffucci's syndrome have multiple enchondromas.3,11
Most benign cartilage tumors do not undergo malignant transformation; however, there is an increased incidence of chondrosarcoma in patients with syndromes involving multiple benign cartilage lesions.3,8,11 Rarely, a solitary enchondroma can transform into a chondrosarcoma.8 Dedifferentiated chondrosarcoma is a rare lesion where a benign cartilage tumor or low-grade chondrosarcoma is juxtaposed on a high-grade spindle cell sarcoma with the histological appearance of an osteosarcoma, malignant fibrous histiocytoma or fibrosarcoma.4,16 Patients with dedifferentiated chondrosarcoma have an extremely poor prognosis and usually die of metastatic disease to the lungs.7,14
Chondrosarcomas can also occur de novo and span a spectrum of histological grades. Recommendations for treatment of patients with low-grade chondrosarcoma have varied in the existing literature.1,13,18 In part this stems from a disparity in the grading systems used by pathologists at different institutions.2,5,10,17,22 The histological criteria for grading malignant cartilage tumors is based on a 3-tiered system.12 Dedifferentiated chondrosarcoma is sometimes referred to as a grade 4 lesion. Because the same lesion may be graded differently at different institutions, many orthopaedic surgeons rely more heavily on radiographic criteria to guide treatment. In general there has been a trend toward more intralesional treatment of grade 1 chondrosarcoma in the extremities in an effort to avoid the functional limitations often present after a major resection and reconstruction.1,13
We present the case of a patient with a low-grade chondrosarcoma in the left medial femoral condyle treated with intralesional surgery. The tumor recurred locally and eventually transformed into a dedifferentiated chondrosarcoma putting her limb and life in jeopardy. The patient's presenting complaints, physical examination, radiographic and histological findings, and treatment course are discussed.
CASE REPORT
A thirty-eight year old female marathon runner presented to an orthopaedic surgeon with persistent medial knee pain three weeks after falling on her left knee. Prior to her fall she had intermittent left knee pain which she attributed to strenuous training. Radiographic workup included plain radiographs (not shown), bone scan and a magnetic resonance image (MRI) of the left knee and distal femur (Figure 1). A lytic lesion with anterior cortical disruption, seen best on the MRI scan, was noted in the medial femoral condyle. Her surgeon felt the lesion was consistent with a probable enchondroma and performed a curettage and bone grafting with iliac crest graft. The patient did well postoperatively and began distance running after three months without difficulty. Two months later, she noted a mass at the medial aspect of the left knee, but repeat imaging studies did not show obvious recurrent tumor.
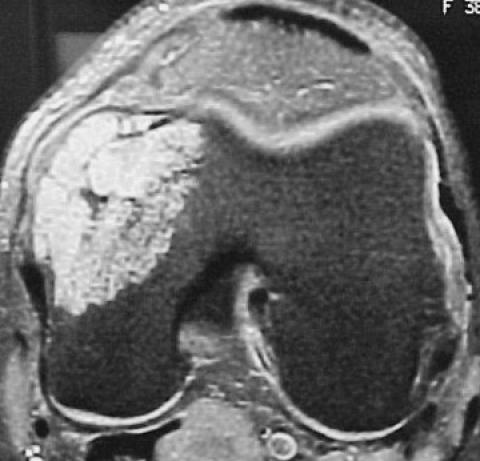
Figure 1.
Coronal T1-weighted (A) and axial T2-weighted (B) MR images demonstrate cortical breakthrough and soft tissue extension of the tumor. This appearance indicates a more aggressive lesion and is consistent with a chondrosarcoma.
Figure 1A.

Figure 1B.
Because of the mass, the patient's initial pathology slides were reviewed at the Mayo Clinic in Rochester, Minnesota where the diagnosis of grade 1 chondrosarcoma was made. The patient was referred to the authors' institution where confirmation of a low-grade (grade 1) chondrosarcoma was made (Figure 2). Histologically the initial tumor was composed of minimally hypercellular, disorganized hyaline cartilage. Although there was minimal variation in cell size and shape (i.e., minimal pleomorphism), the individual cells had evidence of increased nuclear detail and more than occasional binuclear cells were present.
Figure 2.

Low-grade (i.e., grade 1) histology is shown of the initial curetted specimen. The tumor is composed of disorganized, somewhat hypercellular hyaline cartilage. Some cells have an increase in nuclear detail, and there are more than occasional binuclear cells present. Mitotic index: <1 mitosis per 10 high power fields.
At that time the patient had a palpable firm mass on the medial aspect of the left knee that was nonmobile and nontender. There was no inguinal or popliteal adenopathy. Her range of knee motion, ligamentous integrity, strength, and neurologic examinations were normal. Despite the fact that there was no definite recurrence on the imaging studies, it was recommended that the patient undergo resection of the medial femoral condyle and reconstruction with a hemiosteoarticular allograft to remove any residual chondrosarcoma and decrease the likelihood of local recurrence. Being an avid runner and unwilling to give up high impact-loading activities, the patient refused further operative treatment at this time.
One year after consultation at our institution and eighteen months after her initial surgery, the patient developed an obvious radiographic recurrence of the chondrosarcoma with interval growth appreciated on serial plain radiographs and MRI scans (not shown). Again, a wide resection of the condyle was recommended with hemi-osteoarticular allograft reconstruction, but the patient refused due to an unwillingness to accept any modification of her activities. She was treated with a second curettage and bone grafting procedure by her initial surgeon two months later. Later review of that tumor specimen at our institution revealed a tumor largely similar to the initial lesion, except that the degree of cellularity, atypia, and pleomorphism was more pronounced. The matrix was no longer well-formed pure hyaline cartilage, but a mixture of hyaline and myxoid matrix. As a result of these changes, the diagnosis progressed to grade 2 chondrosarcoma (Figure 3). At this point, there was no evidence of a non-cartilaginous, high-grade component.
Figure 3.

Grade 2 chondrosarcoma shown at the time of first relapse. The tumor is composed of disorganized, hypercellular cartilage with increased nuclear detail and more than occasional binuclear cells. However the magnitude of these changes is significantly increased, and there is a higher degree of cellular atypia and pleomorphism: Mitotic index: <2 mitoses per 10 high power fields.
Eleven months after the second curettage and thirty-one months after the initial curettage, the patient developed a rapidly growing mass along the medial aspect of the left knee. Radiographically, the appearance was that of an aggressive high-grade lesion (Figures 4 and 5). A needle biopsy was performed, and the resulting histological findings were those of a dedifferentiated chondrosarcoma. A distal femoral resection and reconstruction with a modular hinged knee prosthesis was performed (Figure 6). At this point, the morphological features of the tumor had dramatically changed. The predominant histological picture was that of a highly anaplastic, spindle-cell tumor with foci of osteoid production; high-grade fibroblastic osteosarcoma. There were also discrete islands of tumor present with a histological appearance of low-grade chondrosarcoma. This juxtaposition of low-grade cartilaginous foci on highgrade spindle-cell sarcoma is diagnostic of dedifferentiated chondrosarcoma (Figure 7).
Figure 4.
Anteroposterior (A) and lateral (B) radiographs of the left distal femur 31 months after the initial surgical procedure showing an aggressive lesion with extensive intramedullary and extraosseous components.
A.

B.

Figure 5.
Coronal T1-weighted (A) and axial T2-weighted (B) MR images demonstrate the extent of the marrow involvement in the distal femur.
A.

B.

Figure 6.
Anteroposterior (A) and lateral (B) radiographs after resection of the distal femur and reconstruction with a modular rotating hinge prosthesis.
A.
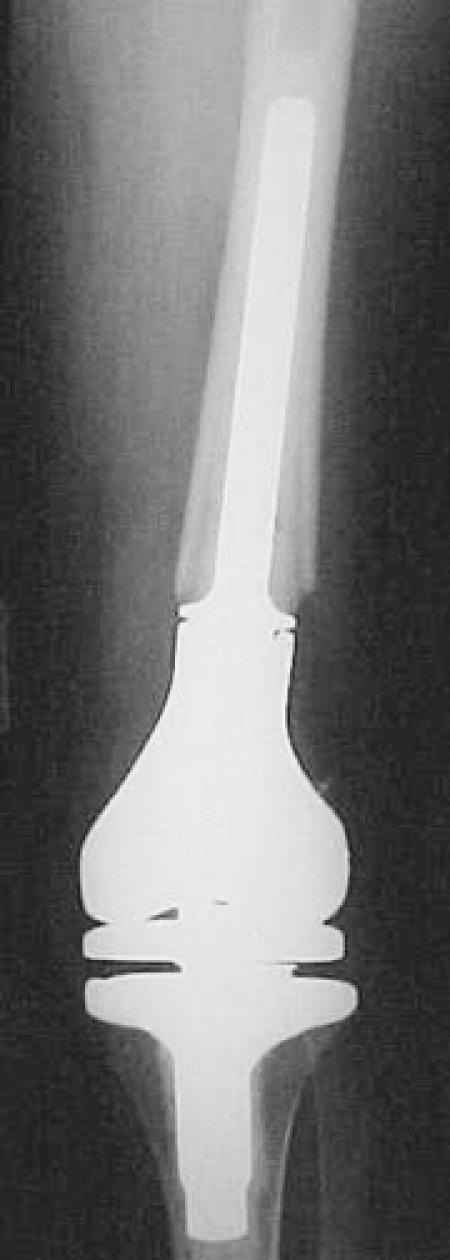
B.

Figure 7.

Dedifferentiated chondrosarcoma from the femoral resection; second local relapse. There is a juxtaposition of well-defined low-grade chondrosarcoma and a highly anaplastic malignant spindle-cell tumor. In other areas (not depicted) there was evidence of osteoid and bone production by the high-grade neoplastic cells.
Staging studies revealed bilateral pulmonary metastases. The patient returned for systemic treatment at our institution, and intravenous chemotherapy with Adriamycin and Cisplatin was initiated. She received three courses of treatment prior to bilateral staged thoracotomies. There were fourteen pulmonary nodules on the left with necrosis of the tumors from 10-50%. On the right there were five nodules with necrosis rates ranging from 25-80% in addition to areas that appeared completely necrotic as a result of prior treatment with chemotherapy. It is of interest that the viable tumor was entirely composed of intermediate- to high-grade chondrosarcoma. The only evidence of the non-chondroid component was fibrosis and granulation tissue, presumed to reflect the results of successful preoperative therapy on the high-grade osteosarcoma component.
Several weeks later the patient noted fullness in the left popliteal fossa, and a needle biopsy of this area revealed local recurrence of the chondrosarcoma. Despite two additional cycles of chemotherapy with high dose ifosfamide, there was continued growth of the popliteal mass. The patient also developed pain and swelling over the dorsum of the left foot. Imaging studies revealed a soft tissue mass dorsal to the navicular bone. It was indeterminate whether this mass represented a soft tissue metastasis or a focus of infection that developed when the patient was neutropenic after chemotherapy. The patient opted for an above knee amputation for local control (Figure 8). Interestingly, the histological review of the locally recurrent popliteal mass revealed progression to a grade 3 chondrosarcoma with no areas of the prior dedifferentiated chondrosarcoma (Figure 9). The soft tissue mass in the foot was a focus of metastatic grade 3 chondrosarcoma. The patient currently walks with an above-knee prosthesis and has progressive inoperable lung metastases.
Figure 8.

Gross appearance of the popliteal fossa recurrence bisected to show the myxoid cartilaginous component.
Figure 9.

The histological appearance of the local recurrence in the popliteal fossa revealed grade 3 chondrosarcoma. The chondroid matrix has myxoid qualities and there is extreme hypercellularity with peripheral spindling of the neoplastic cells. There is marked anaplasia with bizarre giant cell forms and 3 mitoses in this single field. There is no evidence of the high-grade non-cartilaginous component of the prior relapse.
DISCUSSION
The differentiation between a benign cartilage lesion and a low-grade chondrosarcoma can be extremely difficult both clinically and histologically.5,17,22 Although there are established criteria for assessing chondroid aggressiveness in biopsy specimens, the histopathology is only one piece of the puzzle. It is imperative to correlate the clinical and radiographic features of a bony cartilage lesion in order to determine the optimal course of clinical treatment.22 In general, benign cartilage lesions are asymptomatic and often noted incidentally on radiographs obtained after injury to the extremity. Persistent pain prior to the injury might signify an actively growing lesion. The radiographs must be inspected carefully for signs of cortical destruction or periosteal reaction that would raise concerns of malignancy. Even if the cartilage tumor is deemed radiographically quiescent, the patient should be followed clinically with serial radiographs to determine if there is active growth of the lesion over time.
Plain radiographs of benign cartilage lesions show no signs of aggressive growth or destruction of the surrounding cortex.6 Specifically, enchondromas are usually found in the diaphysis or metadiaphysis of long bones or small tubular bones. They have the characteristic appearance of variably calcified cartilage (i.e., so called "smoke ring," or "popcorn" calcification) within the medullary canal. Minimal scalloping of the surrounding cortex is allowed, but there should be no periosteal reaction or cortical breakthrough. The latter findings are suggestive of a chondrosarcoma and should be treated in a more aggressive fashion. A standard bone scan is not ideal for differentiating enchondromas from chondrosarcomas as there is increased technetium uptake even for benign lesions. A computed tomography (CT) scan is helpful to confirm the chondroid nature of the lesion as it can detect the presence of mineralized matrix and better define the extent of endosteal scalloping.6 MRI scans are the most accurate way to determine the extent of marrow involvement or the presence of a soft tissue mass. If the plain radiographs show a classical appearance of a benign cartilage entity, an MRI scan is not necessary.
An accurate and reproducible method of differentiating benign cartilage tumors from low-grade chondrosarcomas is necessary. Radiographic techniques such as dynamic thallium scans or fast contrast-enhanced MRIs may aid in this dilemma.9 Molecular assays for the presence or overexpression of Ki-67 or the platelet-derived growth factor -a (PDGF-a) receptor may also be clinically relevant in the future.21,23 Further work studying matched samples of benign and low-grade malignant lesions may yield information on differentially expressed genes that could be used as markers for histological progression to higher grade lesions.
The majority of dedifferentiated chondrosarcomas are not usually identified until after malignant transformation to a high-grade sarcoma. It is rare to observe the natural history of a dedifferentiated chondrosarcoma as documented in this report. In this particular case, suboptimal surgical treatment of the low-grade chondrosarcoma, albeit at the patient's request, led to recurrence of the lesion. At the time of the second local recurrence, the lesion transformed to a dedifferentiated chondrosarcoma and metastasized to the lungs.
The authors recommend the following approach for the treatment of low-grade cartilage tumors. If the radiographic appearance is benign, the lesion should be treated nonoperatively with the exception of rare, symptomatic cases that can be treated with intralesional curettage and bone grafting. If the radiographs suggest a more aggressive lesion, it should be treated surgically with or without a prior needle biopsy. The biopsy should only be used to confirm a cartilage phenotype and not to determine the tumor grade; chondrosarcoma may be heterogeneous to the extreme, with a single lesion having areas of grade 1 through grade 3. Clearly there are some grade 1 chondrosarcomas that can be treated by intralesional excision with no adverse sequelae, while others have a higher risk of local recurrence after this approach. The radiographic appearance is paramount in making this determination. Symptomatic cartilage lesions within the diaphysis or metadiaphysis of long bones that show only minimal evidence of aggressive radiographic behavior (thickening of the surrounding cortex, endosteal scalloping) may be potentially treated with a thorough intralesional procedure and followed closely for signs of recurrence. However, if local recurrence after an intralesional procedure would put the patient at risk for an amputation, consideration should be given for initial wide resection. The same lesion in the bony pelvis should be resected rather than curetted to avoid a catastrophic local recurrence in this location. 19 A low-grade chondrosarcoma in an expendable bone such as the proximal fibula should be resected. Extremity lesions associated with cortical breakthrough and a soft tissue mass should be resected. Surgical principles of oncology should be closely followed in terms of incision and drain placement, and use of a tourniquet to avoid a large hematoma with contamination of tissue planes.
In summary, this case illustrates the potential pitfalls of treating cartilage lesions. Careful attention to the clinical and radiographic presentation is important in order to avoid local recurrence, dedifferentiation, and metastasis.
References
- 1.Bauer H, Brosjo O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities. Acta Orthop Scand. 1995;66:283–288. doi: 10.3109/17453679508995543. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–2119. [PubMed] [Google Scholar]
- 3.Cannon S, Sweetnam D. Multiple chondrosarcomas in dyschondroplasia (Ollier's disease) Cancer. 1985;55:836–840. doi: 10.1002/1097-0142(19850215)55:4<836::aid-cncr2820550421>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28:461–466. doi: 10.1002/1097-0142(197108)28:2<461::aid-cncr2820280227>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–831. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Flemming DJ, Murphey MD. Enchondroma and chondrosarcoma. Seminars in Musculoskeletal Radiology. 2000;4:59–71. doi: 10.1055/s-2000-6855. [DOI] [PubMed] [Google Scholar]
- 7.Frassica FJ, Unni KK, Beabout JW, Sim FH. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J Bone Joint Surg. 1986 Oct;68-A:1197–1205. [PubMed] [Google Scholar]
- 8.Garrison RC, Unni KK, McLeod RA, Pritchard DJ, Dahlin DC. Chondrosarcoma arising in osteochondroma. Cancer. 1982;49:1890–1897. doi: 10.1002/1097-0142(19820501)49:9<1890::aid-cncr2820490923>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Geirnaerdt MJA, Hogendoorn PC, Bloem JL, Taminiau AHM, van der oude H. Cartilaginous tumors: Fast contrast-enhanced MR imaging. Radiology. 2000;214:539–546. doi: 10.1148/radiology.214.2.r00fe12539. [DOI] [PubMed] [Google Scholar]
- 10.Gitelis S, Bertoni F, Picci P, Campanacci M. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg. 1981 Oct;63-A:1248–1257. [PubMed] [Google Scholar]
- 11.Lewis R, Ketcham A. Maffucci's syndrome: Functional and neoplastic significance. The Journal of Bone and Joint Surgery. 1973;55-A:1465–1478. [PubMed] [Google Scholar]
- 12.Lichtenstein L, Jaffe HL. Chondrosarcoma of bone. Am J Pathol. 1943;19:553–589. [PMC free article] [PubMed] [Google Scholar]
- 13.Marco RAW, Gitelis S, Brebach GT, Healey JH. Cartilage tumors: Evaluation and treatment. J Am Acad Orthop Surg. 2000;8:292–304. doi: 10.5435/00124635-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mercuri M, Picci P, Campanacci L, Rulli E. Dedifferentiated chondrosarcoma. Skeletal Radiology. 1995;24:409–416. doi: 10.1007/BF00941235. [DOI] [PubMed] [Google Scholar]
- 15.Milgram J. The origins of osteochondromas and enchondromas. A histopathologic study. Clinical Orthopaedics and Related Research. 1983;174:264–284. [PubMed] [Google Scholar]
- 16.Mirra JM, Marcove RC. Fibrosarcomatous dedifferentiation of primary and secondary chondrosarcoma: Review of five cases. J Bone Joint Surg. 1974;56A:285–296. [PubMed] [Google Scholar]
- 17.Mirra J, Gold R, Downs J, Eckardt J. A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones. Clinical Orthopaedics and Related Research. 1985;201:214–237. [PubMed] [Google Scholar]
- 18.Ozaki T, Lindner N, Hillmann A, Rodel R, Blasius S, Winkelmann W. Influence of intralesional surgery on treatment outcome of chondrosarcoma. Cancer. 1996;77:1292–1297. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1292::AID-CNCR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis: A review of sixty-four cases. J Bone Joint Surg. 2001;83A:1630–1642. [PubMed] [Google Scholar]
- 20.Scarborough M, Moreau G. Benign cartilage tumors. Orthopedics Clinics of Nor th America. 1996;27:583–589. [PubMed] [Google Scholar]
- 21.Sulzbacher I, Birner P, Trieb K, Muhlbauer M, Lang S, Chott A. Platelet-derived growth factor-a receptor expression supports the growth of conventional chondrosarcoma and is associated with adverse outcome. Am J Surg Pathol. 2001;25:1520–1527. doi: 10.1097/00000478-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Unni KK. Cartilaginous lesions of bone. J Orthop Sci. 2001;6:457–472. doi: 10.1007/s007760170015. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein L, McCarthy E. Ki-67 immunostaining as a tool in the diagnosis of central cartilage lesions. The Iowa Orthopaedic Journal. 1996;16:39–45. [PMC free article] [PubMed] [Google Scholar]


