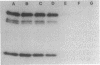
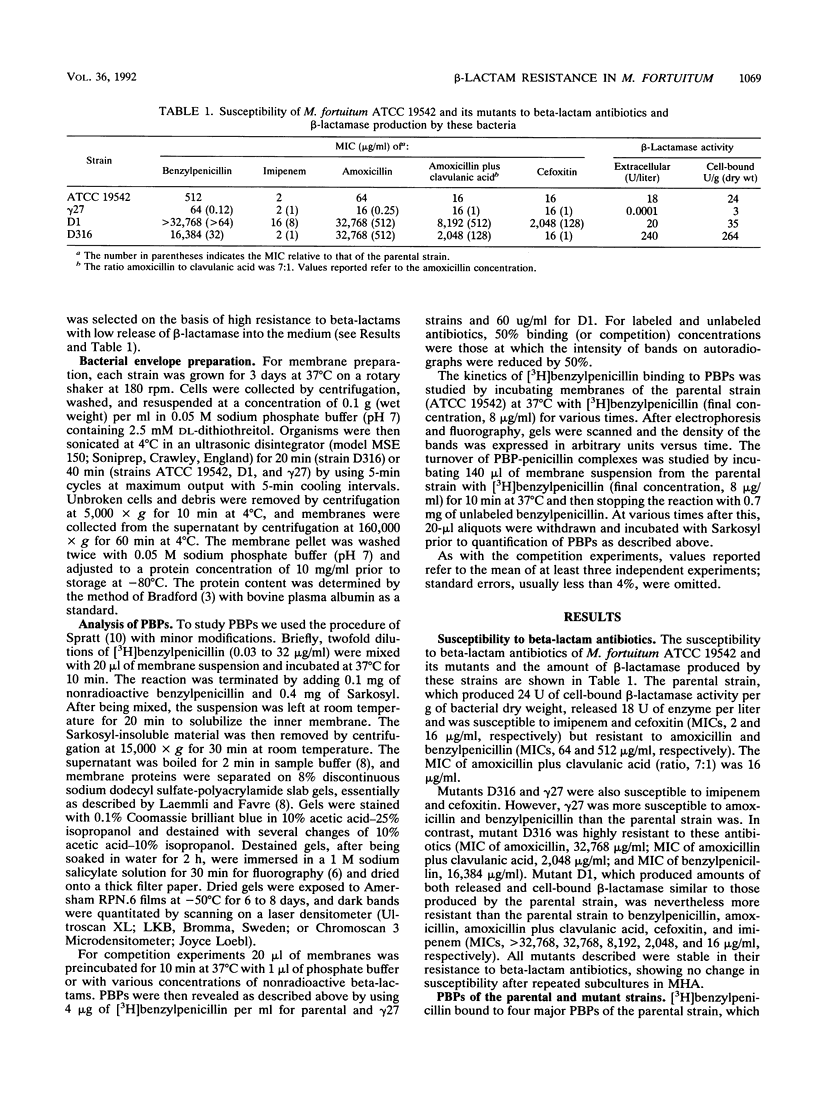
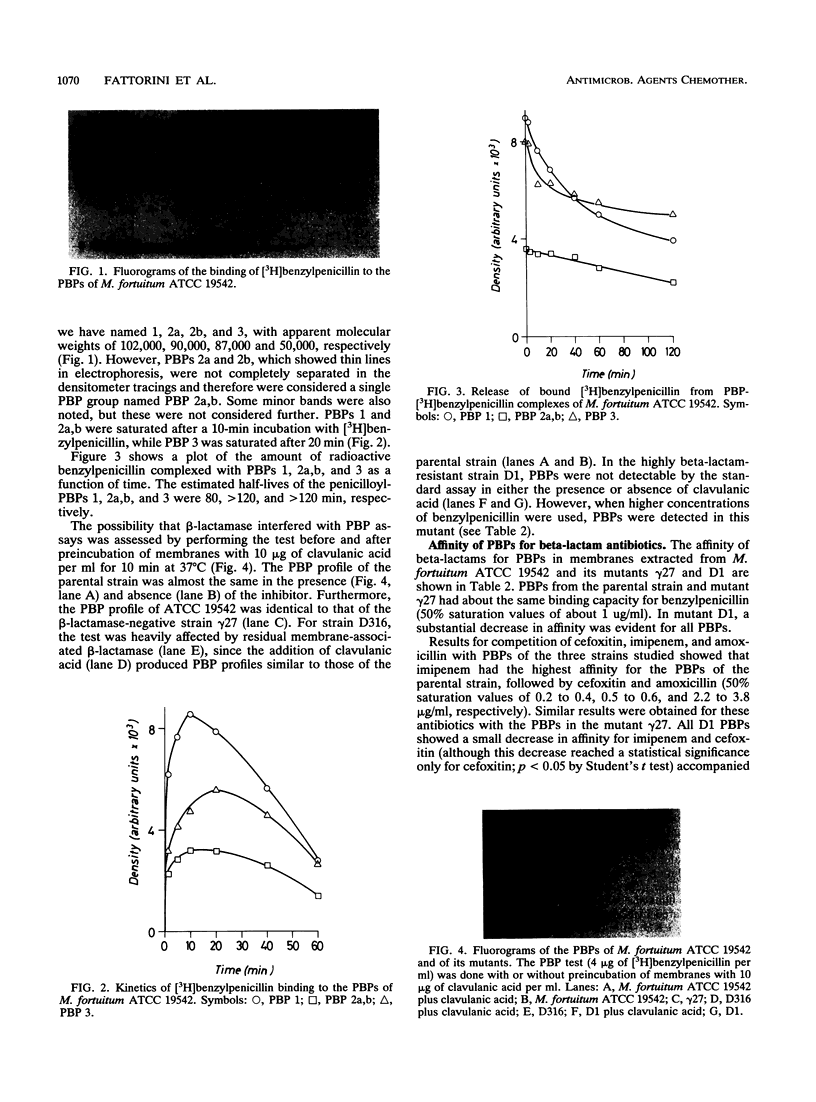
Abstract
It is widely assumed that the high level of intrinsic resistance to beta-lactam antibiotics exhibited by mycobacteria results from the combination of factors including permeability to the drugs, beta-lactamase production, and affinity for penicillin-binding proteins (PBPs). We conducted an evaluation of the second and third factors by isolating nitrosoguanidine-induced mutants from the beta-lactamase-producing strain Mycobacterium fortuitum ATCC 19542 that displayed either elevated or reduced resistance to various beta-lactam antibiotics. The mutants studied included D1 (a beta-lactamase producer with high penicillin resistance), gamma 27 (a low-level beta-lactamase producer with low penicillin resistance), and D316 (a high-level beta-lactamase producer with high penicillin resistance). In all strains examined, four major PBPs, named 1, 2a, 2b, and 3, with apparent molecular weights of 102,000, 90,000, 87,000, and 50,000, respectively, were found. The MICs of various beta-lactams toward ATCC 19542 and its mutants were considered in the context of beta-lactamase production, the quantity of PBPs synthesized, and their affinities for beta-lactam antibiotics. The data obtained show that beta-lactamase production is likely to be an important factor in the expression of resistance by clinical isolates and that PBP alterations can contribute to resistance at least in laboratory-derived mutants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amicosante G., Franceschini N., Segatore B., Oratore A., Fattorini L., Orefici G., Van Beeumen J., Frere J. M. Characterization of a beta-lactamase produced in Mycobacterium fortuitum D316. Biochem J. 1990 Nov 1;271(3):729–734. doi: 10.1042/bj2710729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fattorini L., Amicosante G., Fiorentino D., Franceschini N., Di Marzio L., Oratore A., Orefici G. Inhibitors and inactivators of beta-lactamase from Mycobacterium fortuitum. J Chemother. 1989 Oct;1(5):293–297. doi: 10.1080/1120009x.1989.11738911. [DOI] [PubMed] [Google Scholar]
- Fattorini L., Scardaci G., Jin S. H., Amicosante G., Franceschini N., Oratore A., Orefici G. Beta-lactamase of Mycobacterium fortuitum: kinetics of production and relationship with resistance to beta-lactam antibiotics. Antimicrob Agents Chemother. 1991 Sep;35(9):1760–1764. doi: 10.1128/aac.35.9.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlier V., Gutmann L., Nikaido H. Interplay of cell wall barrier and beta-lactamase activity determines high resistance to beta-lactam antibiotics in Mycobacterium chelonae. Antimicrob Agents Chemother. 1991 Sep;35(9):1937–1939. doi: 10.1128/aac.35.9.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y., Ogawa M., Udou T. Morphological changes induced by beta-lactam antibiotics in Mycobacterium avium-intracellulare complex. Antimicrob Agents Chemother. 1985 Apr;27(4):541–547. doi: 10.1128/aac.27.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Penicillin-binding proteins and the antibacterial effectiveness of beta-lactam antibiotics. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S260–S278. doi: 10.1093/clinids/8.supplement_3.s260. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Bullen M. G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985 Sep;152(3):500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Good R. C., Tschen J. A., Stone M. S. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983 Jul-Aug;5(4):657–679. doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]