Abstract
We have previously shown, by using the phosphate-dependent anti-τ antibodies Tau-1 and PHF-1, that heat shock induces rapid dephosphorylation of τ followed by hyperphosphorylation in female rats. In this study, we analyzed in forebrain homogenates from female Sprague–Dawley rats the activities of extracellular signal regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinase (JNK), glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (Cdk5), cAMP-dependent protein kinase A (PKA), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) at 0 (n = 5), 3 (n = 4), 6 (n = 5), and 12 (n = 5) h after heat shock and in non-heat-shocked controls (n = 5). Immunoprecipitation kinase assays at 0 h showed suppression of the activities of all kinases except of GSK-3β, which showed increased activity. At 3–6 h, the activities of ERK1/2, JNK, Cdk5, and GSK-3β toward selective substrates were increased; however, only JNK, Cdk5, and GSK-3β but not ERK1/2 were overactivated toward purified bovine τ. At 3–6 h, kinase assays specific for PKA and CaMKII showed no increased activity toward either τ or selective substrates. All of eight anti-τ antibodies tested showed dephosphorylation at 0 h and hyperphosphorylation at 3–6 h, except for 12E8, which showed hyperphosphorylation also at 0 h. Immunoblot analysis using activity-dependent antibodies against ERK1/2, JNK, and GSK-3β confirmed the above data. Increased activation and inhibition of kinases after heat shock were statistically significant in comparison with controls. Because τ is hyperphosphorylated in Alzheimer disease these findings suggest that JNK, GSK-3β, and Cdk5 may play a role in its pathogenesis.
The cause of Alzheimer disease (AD) is not known, but it appears to be a syndrome resulting from an interplay of a genetic predisposition, environmental stress factors, and the aging process. Specific genetic defects have been identified on the genes of presenilins 1 and 2 and amyloid precursor protein, but familial AD accounts for less than 5% of all cases (1). Recently, mutations of the τ gene have been described in a group of neurodegenerative dementias (2), but no mutations of the τ gene have been found in AD. The histologic hallmarks of AD are the senile plaques made of extracellular Aβ amyloid and dystrophic neurites and the intraneuronal neurofibrillary tangles (NFT) composed of bundles of abnormal filaments, the so-called paired helical filaments (PHF) and the related straight filaments, the major component of which is hyperphosphorylated τ (3–5). However, the earliest manifestation of hyperphosphorylated τ is a granular form in the somatodendritic compartment (4, 5). Normal fetal and adult brain τ are groups of multiply phosphorylated and thermostable proteins generated by alternative splicing of exons 2 and 3 in the amino-terminal region and exon 10 in the microtubule-binding region of the single τ gene located on chromosome 17q21 (2, 6). Thus, six human (7, 8), four bovine (8, 9), and three rat (8, 10) τ isoforms are generated. Bovine τ isoforms contain either 3 or 4 repeats and 1 or 2 amino-terminal inserts. All three adult rat τ isoforms contain 4 repeats and 0, 1, or 2 amino-terminal inserts. In attempts to create animal models of AD, transgenic mouse models overexpressing 3- or 4-repeat τ isoforms have been developed (11, 12), but the results were rather disappointing because axonal swellings were the main abnormality. These swellings are reminiscent of the β,β′-iminodipropionitrile intoxication, where distal dissociation of microtubules from neurofilaments precedes the formation of proximal axonal swellings (13), suggesting that overexpressed τ interferes with the microtubule–neurofilament interactions. Although original studies localized τ only in axons (14), τ was subsequently also found in the somatodendritic compartment and in glial cells (15). Thus, mere localization of τ in the somatodendritic compartment should not be considered pathological. However, in a transgenic mouse model of frontotemporal dementia and parkinsonism linked to chromosome 17 expressing P301L mutant τ, NFT were found mostly in infratentorial brain and spinal cord but not in cerebral cortex and hippocampus (16).
Although many kinases and phosphatases have been shown to act on τ in vitro (17), the dysregulated processes of dephosphorylation–hyperphosphorylation that lead to PHF-τ are not known. However, after studying the morphology, evolution, and distribution of τ immunoreactivity in AD we suggested that it could represent a defensive response to various stressful stimuli, either endogenous or exogenous (4, 5). To test this hypothesis, we have shown by using the anti-τ antibodies Tau-1 and PHF-1 that heat shock induces rapid dephosphorylation followed by hyperphosphorylation of τ in female rats that is similar to PHF-τ in AD (18, 19), and androgens, but not estrogens, prevent the heat shock-induced hyperphosphorylation of τ (20, 21). The relevance of this model to AD is further enhanced by the many studies that show a stress response in AD brain (22, 23). In this study, to characterize further this rat heat shock model, we investigated in immunocomplex kinase assays the activities of extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun NH2-terminal kinase (JNK), glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (Cdk5), cAMP-dependent protein kinase (PKA), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) in control and heat-shocked female rats.
Materials and Methods
Purification of Bovine τ.
Bovine forebrains were homogenized 1:1 (wt/vol) in 100 mM Hepes, pH 6.9, containing 1 mM EGTA, 0.5 mM EDTA, 0.5 mM MgSO4, 80 mM NaCl, 10 mM Na4P2O7, 20 mM NaF, 0.2 mM Na3VO4, 0.1 mM PMSF, and 10 mM benzamidine. DTT and NaCl were added to a concentration of 2 mM and 0.75 M, respectively, and the mixture was centrifuged at 100,000 × g for 60 min at 4°C. The supernatant was sequentially boiled, taken to 2.5% HClO4, subjected to precipitation with 60% saturated (NH4)2SO4, and centrifuged at 35,000 × g for 30 min at 4°C between each step. The pellet was homogenized in 5 ml of H2O and dialyzed into Mono-S column starting buffer (50 mM Hepes, pH 6.9/1 mM EDTA/1 mM DTT). FPLC was performed with a linear gradient of 50 ml from 0 to 0.5 M NaCl. τ-containing fractions were dialyzed and stored at −80°C.
Heat Shock.
Two- to three-month-old female Sprague–Dawley rats were heat-shocked as described (19).
Protein Extraction for Kinase Assays and Immunoblot Analysis.
In brief, 0, 3, 6, and 12 h after heat shock, rats were killed by intracardiac perfusion of 100 mM Tris⋅HCl, pH 7.6/6 mM EGTA at 4°C. The forebrain was homogenized in extraction buffer [50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/1 mM EGTA, containing protease inhibitors (1 mM PMSF and 1× Complete protease inhibitor mixture from Boehringer Mannheim) and phosphatase inhibitors (1 mM Na3VO4/1 mM NaF/2.5 μM microcystin LR/2 μM okadaic acid)] and centrifuged twice at 100,000 × g for 1 h at 4°C. The supernatant was stored at −80°C. Protein content was estimated by the Bradford method.
Immunocomplex Protein Kinase Assays (ERK1/2, JNK, Cdk5, and GSK-3β).
Briefly, 200 μg of protein extract was precleared with 20 μl of protein G-agarose beads for 1 h at 4°C. Antibodies specific for ERK1/2 (Upstate Biotechnology, cat. no. 06–182), JNK1 (PharMingen, clone G151–133), and Cdk5 (Santa Cruz Biotechnology, C-8) were added and incubated for 2 h at 4°C, followed by an additional incubation with protein G-agarose beads. The immunocomplexes were washed three times with the extraction buffer containing 1% Triton X-100 and twice in kinase buffer (20 mM Hepes, pH 7.4/20 mM MgCl2/20 mM β-glycerophosphate/0.1 mM Na3VO4/2 mM DTT). The washed immunocomplexes were incubated with 20 μl of kinase buffer containing myelin basic protein (MBP; 5 μg), or c-Jun-GST (1 μg; GST is glutathione S-transferase) or histone H1 (5 μg), respectively, and [γ-32P]ATP [20 μCi (1 μCi = 37 kBq) per tube, 50 μM final concentration] for 20 min at 30°C. The phosphorylation reactions were terminated by boiling in 20 μl of 2× Laemmli sample buffer (24). Eight microliters (1 μg each of MBP and histone H1 or 0.2 μg of c-Jun-GST) was resolved on SDS/10% PAGE, followed by autoradiography. Phosphorylated bands were excised and quantified by liquid scintillation counting. For GSK-3β assay, 100 μg of protein extract was precleared with 20 μl of protein A-agarose beads for 1 h at 4°C. One microgram of antibody against GSK-3β (Transduction Laboratories, Lexington, KY) was added and incubated for 1 h at 4°C, followed by an additional incubation with protein A-agarose beads. The immunocomplexes were washed three times with the extraction buffer containing 1% Triton X-100 and twice in GSK-3β kinase buffer (250 mM sodium glycerophosphate, pH 7.4/1 M NaCl/100 mM MgCl2/5 mM EGTA/5 mM benzamidine/0.5 mM Na3VO4/5 mM DTT), and incubated with 20 μl of kinase buffer containing 62.5 μM phosphoglycogen synthase peptide 2 (PGSP-2) and [γ-32P]ATP (20 μCi per tube, 50 μM final concentration). After 10 min of incubation at 30°C, the reaction mixture was spotted onto a P-81 filter and the radioactivity determined as described (25). For determination of ERK1/2, JNK, Cdk5, and GSK-3β activity toward τ, the selective substrates were replaced with 5 μg of bovine τ. Control reactions were performed in the absence of antibodies.
PKA Assay.
The reaction mixture contained 10 μg of extract protein, 50 mM Hepes at pH 7.4, 1 mM DTT, 10 mM MgCl2, 10 μM cAMP, 50 μM Kemptide, and [γ-32P]ATP (1 μCi per tube, 50 μM final concentration) in a final volume of 40 μl. A control reaction was performed in the presence of 10 μM PKI, an inhibitory peptide specific for PKA. After incubation at 30°C for 5 min, the reaction was terminated by spotting a 20-μl aliquot on P-81 filter paper. The radioactivity was determined as described (25). To measure the PKA activity toward τ, Kemptide was replaced with 2 μg of bovine τ as substrate. Two control reactions were also performed by either omitting τ or adding 10 μM PKI. After incubation at 30°C for 2 min, the reaction was terminated by spotting 20 μl on GF/C filters and the 32P incorporation was measured by scintillation counting. The radioactivity associated with the two control reactions was subtracted to obtain the 32P incorporation into bovine τ.
CaMKII Assay.
The reaction mixture contained 10 μg of extract protein, 50 mM Hepes (pH 7.4), 1 mM DTT, 10 mM MgCl2, 1 mM CaCl2, 2 μM calmodulin, 50 μM autocamtide-2 and [γ-32P]ATP (1 μCi per tube, 50 μM final concentration) in a final volume of 40 μl. A control reaction was performed in the presence of 10 μM autocamtide-2-related inhibitory peptide to specifically inhibit CaMKII activity. After incubation at 30°C for 2 min, the reaction was terminated and the radioactivity was determined as for the PKA assay. To measure the CaMKII activity toward τ, the autocamtide-2 was replaced with 2 μg of bovine τ. Two control reactions were also performed by either omitting τ or adding 10 μM autocamtide-2-related inhibitory peptide. After incubation at 30°C for 2 min, the reaction was terminated and the radioactivity on the filters was counted as for the PKA assay. The radioactivity associated with the two control reactions was subtracted to obtain the 32P incorporation into bovine τ.
Statistical Analysis.
Statistical analysis was performed by one-way analysis of variance (ANOVA). Significant ANOVA (P < 0.05) was followed by the Tukey test of pairwise multiple comparisons.
Immunoblot Analysis of Activated ERK1/2 and JNK and Inactivated GSK-3β.
Phosphospecific antibodies against activated ERK1/2 and JNK and inactivated GSK-3β were used in immunoblots of SDS/10% PAGE. Incubations in primary antibody were done overnight at 4°C and in goat anti-rabbit IgG coupled to horseradish peroxidase for 1 h at room temperature. Enhanced chemiluminescence was used as the detection system.
Immunoblot Analysis of τ and Heat Shock Proteins in SDS Cerebral Extracts.
SDS cerebral extracts were prepared as described (18, 19). Fifty micrograms of protein of SDS extracts from control and heat-shocked rats was electrophoresed in 5–12.5% linear gradient polyacrylamide gels, transferred to nitrocellulose membrane, and immunostained as above. The following eight anti-τ antibodies were used: Tau-1, PHF-1, 12E8, and AT270 which require nonphosphorylated Ser195/198/199/202 (26), phosphorylated Ser396 and Ser404 (27), phosphorylated Ser262 and/or to a lesser extent Ser356 (28), and phosphorylated Thr181 (29), respectively; Tau-2, which is PHF-conformation dependent (30); SMI34, which is PHF-conformation dependent in cooperation with unidentified phosphorylation sites (31); and the phosphate-independent Tau-5 (32) and Tau-46, which recognize the sequences Ser210-Arg230 and Leu428-Leu441, respectively (33). Antibodies against the heat shock proteins Hsc70 (clone 1B5) and Hsp72 (clone C92F3A5) were purchased from StressGen Biotechnologies (Victoria, BC, Canada). Anti-mouse IgG coupled to horseradish peroxidase was used as secondary antibody. All primary and secondary incubations were for 1 h except for Tau-2, which required overnight incubation.
Results
Purification of τ.
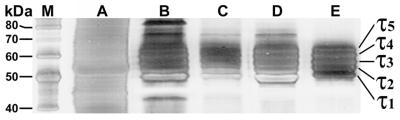
τ was isolated to near homogeneity so that even after overloading (5 μg of τ) and silver staining of SDS/PAGE gels no other proteins were detectable except of the five major peptides of native bovine brain τ (labeled τ1−τ5; Fig. 1, lane E). Because four τ isoforms are alternatively spliced from a single bovine τ gene (8, 9), the presence of a fifth peptide indicated, as expected, some in vivo phosphorylation.
Figure 1.
SDS/PAGE gels stained with silver show the purification steps of bovine τ. Lanes: M, molecular mass standards; A, crude extract; B, after HClO4 precipitation; C, after (NH4)2SO4 precipitation; D, after dialysis in FPLC column starting buffer; and E, purified τ (5 μg).
Immunoblot Analysis of τ and Heat Shock Proteins.
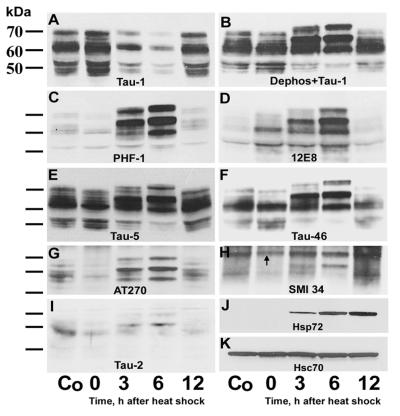
In immunoblots of SDS cerebral extracts from control and heat-shocked rats (Fig. 2), there was dephosphorylation and hyperphosphorylation of τ at 0 and 3–6 h after heat shock, respectively. The only exception was observed with 12E8 which, in addition to 3 and 6 h, showed τ hyperphosphorylation also at 0 h after heat shock (Fig. 2D). Dephosphorylation of τ at 0 h was evidenced by accentuation and attenuation of τ isoforms recognized by Tau-1 and PHF-1, AT270, SMI34, and Tau-2, respectively. Hyperphosphorylation of τ peaked at 6 h and was exemplified by upward gel mobility shift with resultant changes in banding patterns; it was recognized by Tau-1 after alkaline phosphatase treatment (Fig. 2B), PHF-1, AT270, 12E8, Tau-5, and Tau-46. Hyperphosphorylation at 3 and 6 h involved already-existing τ as was evidenced by a decrease in staining intensity of all three τ isoforms revealed by Tau-1 (Fig. 2A) and the 52-kDa isoform revealed by Tau-5 (Fig. 2E) and Tau-46 (Fig. 2F), indicating a precursor–product relationship. The PHF conformation of τ was evident at 3 and 6 h with Tau-2 (Fig. 2I) and SMI34 (Fig. 2H). Similar results were obtained when the kinase assay extraction buffer was used instead of SDS.
Figure 2.
Immunoblots of SDS cerebral extracts in control (Co) and heat-shocked rats probed with antibodies against τ (A–I) or heat shock proteins (J and K) 0–6 h after heat shock. Note: (i) the accentuation in A and attenuation in C and G–I of τ polypeptides at 0 h; (ii) the accentuation but no gel mobility shift of τ isoforms at 0 h in D; (iii) the upward gel mobility shift and accentuation of τ polypeptides at 3 and 6 h in B–I; (iv) the attenuation of all three τ isoforms in A and the attenuation of the 52-kDa τ isoform in B, E, and F at 3 and 6 h indicating a precursor–product relationship caused by hyperphosphorylation; and (v) the appearance at 3 h and progressive accentuation between 6 and 12 h of the heat shock-induced Hsp72 in J while the amount of the constitutively expressed Hsc70 remained unchanged K. The arrow in H points to the low molecular weight subunit of neurofilaments. Dephos, dephosphorylation of transferred proteins with alkaline phosphatase before immunostaining.
While there was no difference in the amount of constitutive Hsc70 between control and heat-shocked rats (Fig. 2K), the inducible Hsp72 was absent from controls but in heat-shocked rats became detectable at 3 h and increased progressively between 6 and 12 h after heat shock (Fig. 2J).
Initial Rate Kinase Assays.
For all kinase assays the concentration of τ and selective substrates were above saturation level, as determined in preliminary experiments, so that the initial reaction rate was proportional to enzyme activity alone, linear within the time of assay, and, thus, physiologically relevant. The results for all six kinase assays toward τ (Fig. 3) and their selective substrates (Fig. 4) are summarized in Table 1 and individually analyzed below.
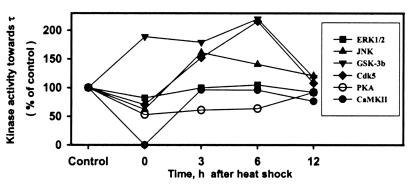
Figure 3.
Relative initial rate kinase activities toward τ of each of the six kinases—i.e., ERK1/2, JNK, GSK-3b, Cdk5, PKA, and CaMKII in control (non-heat-shocked) and heat-shocked rats are compared after normalization to their respective control (100). Note: (i) the decreased activity of all kinases at 0 h after heat shock except GSK-3β, which was increased; and (ii) the increased activity of JNK, GSK-3β, and Cdk5 but not ERK1/2 at 3 and 6 h after heat shock.
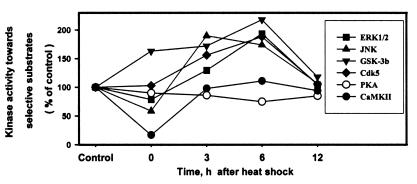
Figure 4.
Relative initial rate kinase activities toward their selective substrates of each of the six kinases—i.e., ERK1/2, JNK, GSK-3b, Cdk5, PKA, and CaMKII in control (non-heat-shocked) and at 0–12 h after heat shock are compared after normalization to their respective control (100). Note: (i) the decreased activity of all kinases at 0 h after heat shock except GSK-3β, which was increased, and Cdk5, which was unchanged; and (ii) the increased activity of JNK, GSK-3β, Cdk5, and ERK1/2 at 3 and 6 h after heat shock.
Table 1.
Initial rate kinase activities towards τ and selective substrates in rat cerebral extracts 0–12 h after heat shock
| Kinase | Substrate |
32P cpm/1 μg
of τ or various amounts of selective substrates*
|
||||
|---|---|---|---|---|---|---|
| Control (n = 4†) | 0 h (n = 4†) | 3 h (n = 3) | 6 h (n = 4†) | 12 h (n = 3) | ||
| ERK1/2 | τ | 2,136 ± 304 | 1,758 ± 209 | 2,135 ± 366 | 2,238 ± 416 | 1,966 ± 376 |
| MBP | 16,477 ± 1,218 | 12,981 ± 2,040 | 21,341 ± 1,432‡ | 31,899 ± 4,881‡ | 17,200 ± 1,620 | |
| JNK | τ | 707 ± 85 | 438 ± 51‡ | 1,138 ± 136‡ | 997 ± 163‡ | 858 ± 90 |
| c-Jun-GST | 3,740 ± 670 | 2,205 ± 470 | 7,100 ± 415‡ | 6,495 ± 1,065‡ | 3,995 ± 835 | |
| GSK-3β | τ | 527 ± 95 | 998 ± 143‡ | 945 ± 137‡ | 1,158 ± 141‡ | 624 ± 98 |
| PGSP-2 | 18,741 ± 2,888 | 30,568 ± 2,564‡ | 32,307 ± 3,636‡ | 40,913 ± 6,330‡ | 22,031 ± 4,025 | |
| Cdk5 | τ | 2,059 ± 227 | 1,444 ± 172 | 3,137 ± 612 | 4,442 ± 886‡ | 2,220 ± 400 |
| Histone H1 | 7,034 ± 963 | 7,223 ± 1,104 | 10,990 ± 2,207‡ | 13,232 ± 1,236‡ | 7,485 ± 1,447 | |
| PKA | τ | 376 ± 108 | 198 ± 68‡ | 230 ± 21 | 239 ± 67 | 345 ± 80 |
| Kemptide | 11,115 ± 1,486 | 9,982 ± 2,749 | 9,510 ± 2,358 | 8,309 ± 1,687 | 9,488 ± 3,247 | |
| CaMKII | τ | 1,548 ± 387 | 1 ± 8‡ | 1491 ± 287 | 1,487 ± 324 | 1,183 ± 166 |
| Autocamtide-2 | 60,576 ± 4,625 | 10,366 ± 2,049‡ | 59,179 ± 7,487 | 67,260 ± 8,901 | 57,157 ± 7,739 | |
MBP, 1 μg; c-Jun-GST, 1 μg; PGSP-2, 3.79 μg; histone H1, 1 μg; Kemptide, 0.7 μg; and autocamtide-2, 1.53 μg. n, number of rats.
For GSK-3β n = 3.
P < 0.05 in comparison with respective controls (Tukey test).
In control non-heat-shocked rats, the activities of the six kinases toward τ in decreasing order of potency after normalizing to 1.00 for PKA were as follows: ERK1/2 (5.68) > Cdk5 (5.48) > CaMKII (4.12) > JNK (1.88) > GSK-3β (1.40) > PKA (1.00) (Table 1). The overall activities of the six kinases toward τ were significantly different (F = 47.267, df = 5, 22; P < 0.001). In multiple comparisons, the kinase activities were significantly higher (P < 0.05) in the following: ERK1/2 vs. all except Cdk5; Cdk5 vs. JNK, GSK-3β, and PKA; and CaMKII vs. JNK, GSK-3β, and PKA. There were no significant differences in potency toward τ among JNK, GSK-3β, and PKA in control rats.
A phosphospecific antibody against activated ERK1/2, a proline-directed kinase, showed increased activation beginning at 3 h and peaking at 6 h, and gradual return to control level of activity by 12–24 h after heat shock (Fig. 5A). At the same time, the total amounts of ERK1/2, as revealed by an activation-independent antibody, remained unchanged (Fig. 5B). Kinase assays, however, showed an almost 2-fold increase in activity toward MBP but not toward τ at 6 h, after mild suppression toward both at 0 h (Table 1; Figs. 3, 4, and 5 C–F). The difference was not significant for τ (F = 1.723, df = 4, 17; P = 0.205) but significant for MBP (F = 27.99, df = 4, 17; P < 0.001).
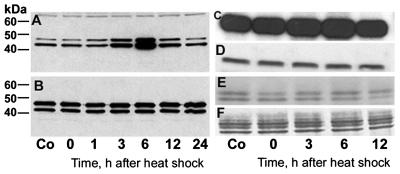
Figure 5.
ERK1/2 shows increased activation after heat shock and hyperphosphorylates MBP but not τ. (A and B) Immunoblots of cerebral extracts from control (Co) and heat-shocked rats probed with the antibodies against activated and total ERK1/2, respectively. The phosphospecific antibody shows increased activation of ERK1/2 at 3 and 6 h after heat shock (A) but the total amount of ERK1/2 is unchanged (B). In A, note the equal loading of extract protein in each lane indicated by the nonspecific staining of a higher molecular weight protein. Audoradiographs (C and E) of Coomassie blue-stained (D and F) SDS/PAGE gels of kinase assay mixtures toward MBP (C and D) and τ (E and F) show hyperphosphorylation of MBP but not τ at 3 and 6 h after heat shock.
When a phosphospecific antibody against activated JNK, another proline-directed kinase, was used, both JNK1 (46 kDa) and JNK2 (54 kDa) showed increased activation at 1–6 h after heat shock. The peak activity occurred earlier than ERK1/2 at 1–3 h and gradually returned to control levels by 12–24 h (Fig. 6A). Again, the total amounts of JNK1/2 were not different from the control (Fig. 6B). However, in contrast to ERK1/2, in kinase assays activated JNK hyperphosphorylated both τ and its substrate c-Jun-GST to 61% and 90% above control levels, respectively, at 3 h after an initial suppression at 0 h (Table 1; Figs. 3, 4, and 6 C–F). The τ1 and τ2 polypeptides were the most heavily phosphorylated (Fig. 6E). The difference was significant for both τ (F = 21.63, df = 4, 17; P < 0.001) and c-Jun-GST (F = 27.267, df = 4, 17; P < 0.001).
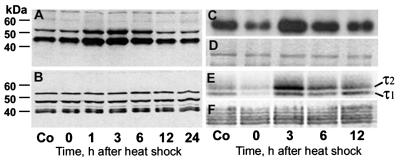
Figure 6.
JNK shows increased activation after heat shock and hyperphosphorylates both c-Jun-GST and τ. (A and B) Immunoblots of cerebral extracts from control (Co) and heat-shocked rats probed with antibodies against activated and total JNK1/2, respectively. The phosphospecific antibody shows increased activation of JNK1/2 at 1–6 h after heat shock (A), but the total amount of JNK1/2 did not change (B). Both antibodies bind nonspecifically to other proteins. Autoradiograph (C and E) of Coomassie blue-stained (D and F) SDS/PAGE gels of aliquots of kinase assay mixtures toward c-Jun-GST (C and D) and τ (E and F) show dephosphorylation and hyperphosphorylation of both at 0 h and 3–6 h after heat shock, respectively.
A phosphospecific antibody against inactivated GSK-3β showed increased activity at 0–6 h after heat shock (Fig. 7A) while the total amounts remained unchanged (Fig. 7B). In kinase assays also, GSK-3β was the only kinase that showed increased activation at 0 h (89% and 63% increase toward τ and PGSP-2, a specific substrate, respectively) in addition to 3 and 6 h (Table 1; Figs. 3, 4, and 7). The τ1−τ3 polypeptides were the most heavily phosphorylated. The more than 2-fold increase in phosphorylation of both τ and PGSP-2 peaked at 6 h (120% and 118% increase above control levels, respectively; Table 1). The difference was significant for both τ (F = 13.564, df = 4, 14; P < 0.001) and PGSP-2 (F = 13.733, df = 4, 14; P < 0.001).
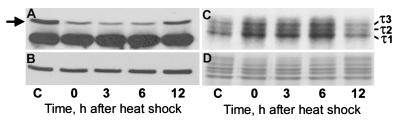
Figure 7.
GSK-3β shows increased activation and hyperphosphorylates τ at 0–6 h after heat shock. (A) Immunoblot of cerebral extracts from control (C) and heat-shocked rats probed with antibodies against inactivated GSK-3β [(phospho-GSK-3β (Ser9)] (arrow) and Cdk5 as a loading control (lower row). Note the decrease in the inactivated form of GSK-3β after heat shock. (B–D) Immunoblot probed with the activation-independent antibody against GSK-3β (B) and autoradiograph (C) of Coomassie blue-stained (D) SDS/PAGE gels of aliquots of kinase assay mixtures show hyperphosphorylation of τ at 0–6 h. Note the equal amounts of GSK-3β (B) and τ (D) in all lanes.
In kinase assays, Cdk5, together with ERK1/2, showed the highest activity toward τ (Table 1). However, in contrast to ERK1/2, Cdk5 hyperphosphorylated τ at 3 and 6 h after heat shock to 53% and 116% above control levels, respectively (Table 1; Fig. 3, and Fig. 8D). The τ2 and τ3 polypeptides were the most heavily phosphorylated. Histone H1, a substrate for Cdk5, was also hyperphosphorylated to 56% and 88% above control levels at 3 and 6 h, respectively (Table 1; Figs. 4 and 8A). At 0 h, however, Cdk5 activity toward τ was decreased 30% below control levels, but remained normal toward histone H1 (Table 1; Figs. 8 A and D). The difference was significant for both τ (F = 18.904, df = 4, 17; P < 0.001) and histone H1 (F = 15.349, df = 4, 17; P < 0.001).
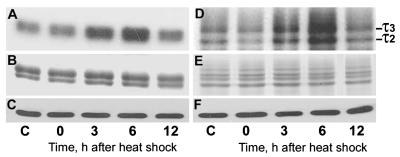
Figure 8.
Cdk5 hyperphosphorylates both histone H1 and τ at 3 and 6 h after heat shock. Autoradiographs (A and D) of Coomassie blue-stained (B and E) and immunoblots probed with the Cdk5 antibody (C and F) of SDS/PAGE gels of aliquots of kinase assay mixtures toward histone H1 (A and B) and τ (D and E) show hyperphosphorylation of both histone H1 and τ at 3 and 6 h after heat shock. Note equal amounts of Cdk5, histone H1, and τ in all lanes. C, control.
At 0–6 h after heat shock, PKA activity toward both τ and Kemptide was lower in heat-shocked than control rats (Table 1; Figs. 3 and 4). Specifically, PKA activity toward τ was 47%, 39%, and 37% below control levels at 0, 3, and 6 h, respectively. The difference was significant for τ (F = 3.87, df = 4, 17; P = 0.028) but not for Kemptide (F = 0.756, df = 4, 17; P = 0.572).
The activity of CaMKII was abolished toward τ and decreased dramatically (83%) toward autocamtide-2 at 0 h after heat shock. It returned to approximate control levels toward both τ and autocamtide-2 at 3–6 h (Table 1; Figs. 3 and 4). The difference was significant for both τ (F = 22.132, df = 4, 17; P < 0.001) and autocamtide-2 (F = 34.442, df = 4, 17; P < 0.001).
The activities of all kinases toward both τ and their selective substrates returned to approximately control levels at 12 h after heat shock.
Correlation Between Immunoblot Analysis of τ and Kinase Activities.
The above kinase assays showed that JNK, GSK-3β, and Cdk5 hyperphosphorylated τ after heat shock. Examination of the epitopes recognized by Tau-1, PHF-1, 12E8, and AT270 (Fig. 2) shows that one or more of these three kinases could be responsible—i.e., all three kinases for the Tau-1 and PHF-1 sites, GSK-3β for the 12E8 site, and JNK and Cdk5 for the AT270 site (17).
Discussion
ERK1/2 is a proline-directed kinase that in vitro phosphorylates τ to the highest stoichiometry (34). In control rats, ERK1/2 activity toward τ was also the highest among the six kinases. However, although it showed increased activation at 3–6 h after heat shock, as was demonstrated by using phosphospecific antibodies and assay toward MBP, it did not hyperphosphorylate τ. This was a surprising finding, but similar dissociation between activation of ERK1/2 and phosphorylation of τ has been described previously (35). It appears, then, that ERK1/2 does not hyperphosphorylate τ after heat shock. It may, however, function as a priming kinase after the initial dephosphorylation of τ.
JNK is another proline-directed kinase that, after certain stresses such as UV irradiation, osmotic stress, and inflammatory cytokines, is activated by dual phosphorylation on Thr and Tyr in the Thr-Pro-Tyr motif (36). However, protein-damaging conditions such as heat shock and oxidative stress activate JNK by a not-yet-known mechanism. JNK has a low constitutive activity and stress does not affect its transcript or protein levels. The low basal activity of JNK appears to be caused by its association with GST Pi (GSTp; ref. 37), and its dephosphorylation and deactivation are perhaps mediated by protein phosphatase 2Cα (38). Upon heat shock, dephosphorylation and inactivation of JNK is specifically suppressed (39), and GSTp undergoes oxidative oligomerization and dissociation from JNK (37). This sequence of events could explain the early activation and peak by 3 h after heat shock. However, its gradual deactivation might be brought about by the inducible Hsp72, which inhibits the suppression of a putative JNK phosphatase (39), and in our model becomes detectable by 3 h after heat shock (Fig. 2J). Thus, as it happens in vitro and in cell cultures (40, 41), JNK plays a role in the vivo hyperphosphorylation of τ.
GSK-3β also phosphorylates proline sites in τ and other proteins (42), but, after a priming phosphorylation, may sequentially phosphorylate Ser/Thr. There are five such motifs in the microtubule-binding repeats of τ (S258KIGS262, S285NVQS289KCGS293, S320KCGS324, and S352KIGS356) that could be phosphorylated. In our studies, GSK-3β showed increased activity at 0–6 h after heat shock and hyperphosphorylated τ and its substrate. It is also noteworthy that GSK-3β was the only kinase that showed increased activity at 0 h. GSK-3β is constitutively active and inhibited by phosphorylation on Ser9. Protein phosphatase 2A, however, which dephosphorylates GSK-3β (43), is activated during heat shock (see below), and may reverse this regulatory inhibition and overactivate GSK-3β. Because GSK-3β plays a major role in neuronal apoptosis (44) and is also preferentially associated with NFT (45), it may be involved in the hyperphosphorylation of τ in AD.
The possibility that Cdk5, a proline-directed Ser/Thr kinase specific for neurons, plays a central role in the neurodegeneration of AD has recently been augmented by cell culture studies which showed that Cdk5 phosphorylates τ, causes cytoskeletal disorganization, and promotes neuronal death (46, 47). Cdk5 associates with microtubules (48) and NFT (45) and phosphorylates τ in vitro (49). In this study, as is the case with ERK1/2, it phosphorylated τ to the highest stoichiometry in control rats, but, in contrast to ERK1/2, at 3–6 h after heat shock it hyperphosphorylated not only its substrate but also τ. Our findings together with the previous studies suggest that Cdk5 may play a pivotal role in the etiopathogenesis of AD.
PKA has been suggested as one of the second messenger-dependent protein kinases that play a direct role in the early stages of neurofibrillary degeneration of AD (50). Also, in sequential phosphorylation studies it generates an AD-specific epitope on τ when preceded by GSK-3β (51) and phosphorylates τ in vitro (52). However, in our heat shock model PKA does not appear to play a role in the hyperphosphorylation of τ.
Like PKA, another second messenger-dependent kinase, CaMKII, phosphorylates τ in vitro (53). In the brain, CaMKII is intricately linked to the induction of long-term potentiation (54), a process thought to contribute to learning and memory. However, in our model it did not hyperphosphorylate τ or its substrate. Remarkably, at 0 h after heat shock CaMKII activity toward τ was abolished. This is perhaps caused by dephosphorylation of Thr286 of CaMKII by protein phosphatases PP2A and/or PP1, which differentially inactivate soluble and postsynaptic density-associated CaMKII, respectively (55).
All of the phosphorylation- and the two conformation-dependent anti-τ antibodies demonstrated dephosphorylation at 0 h and subsequent hyperphosphorylation of τ, except 12E8, which showed hyperphosphorylation also at 0 h after heat shock. This is a very intriguing finding. Both protein phosphatases PP1 and PP2A bound to microtubules (56, 57), but whereas τ anchors PP1 to microtubules (56), PP2A is directly linked to the microtubule-binding region of cytosolic τ by ionic interactions (58). This interaction of τ with PP2A precludes PP2A from dephosphorylating Ser262 and Ser356, because both amino acids are located in the first and fourth microtubule-binding repeats of τ, respectively, which also bind to PP2A and thus cannot be dephosphorylated by PP2A. However, because the catalytic C subunit of PP2A does not interact with τ alone, it remains free to dephosphorylate τ in regions other than its microtubule-binding domain (58). Thus activation of PP2A, perhaps by ceramide (59) formed during heat shock (60), and suppression of all kinases except GSK-3β (see above), which might phosphorylate τ at Ser262, could explain the overall dephosphorylation of τ but hyperphosphorylation at Ser262 and/or Ser356 at 0 h.
JNK, GSK-3β, and Cdk5 phosphorylate τ at the hyperphosphorylated sites detected by the anti-τ antibodies (17), and all three showed increased activity after heat shock. In the literature cited above, there is ample evidence that phosphorylation of τ by Cdk5 and GSK-3β either alone or synergistically inhibits polymerization of tubulin, phosphorylation by Cdk5 detaches microtubule-associated τ and depolymerizes tubulin (61), and all three promote apoptosis and neuronal death. In the rat heat shock model, abundant τ-decorated microtubules are present (19). Also, hyperphosphorylation of τ markedly increases in multiply heat-shocked rats despite the rapid dephosphorylation after each heat shock episode (19). However, no NFT are formed. In contrast, AD is a lifelong process, and a chronic cumulative hyperphosphorylation of τ is perhaps necessary for the formation of NFT in a susceptible host. In AD, the most important τ abnormality is its hyperphosphorylation and aggregation into abnormal filaments. This is the reason it is visible in AD brain either as granular, and perhaps prematurely labeled “pretangle,” form or as PHF (4, 5). Although in in vitro models of PHF formation phosphorylation of τ may not be necessary or sufficient (62), in in vivo models the heavily phosphorylated τ and not the abnormal filaments per se correlate with neuronal degeneration (63). The rat heat shock model in its transient form and lack of PHF formation is certainly not a model of AD, but it allows us to study in just 6 h in a simple and reproducible way the dysregulated processes of dephosphorylation–hyperphosphorylation of τ that lead to AD and, in humans, take a lifetime to develop. Also, the observations that Aβ amyloid deposition may be related to stress (64, 65) enhance the relevance of the heat shock model to AD. However, we have perhaps presented here half of the story. When the analysis also of phosphatases and perhaps more kinases is completed, we envision the rat heat shock model as a useful means to test the effectiveness of various therapeutic protocols for AD.
Acknowledgments
We thank Drs. L. I. Binder, P. Davies, J. Q. Trojanowski, and P. Seubert for supplying the anti-τ antibodies Tau-1, Tau-2, Tau-5, PHF-1, Tau-46, and 12E8. This work was supported by the Alzheimer's Association (S.Ch.P.).
Abbreviations
- AD
Alzheimer disease
- NFT
neurofibrillary tangles
- PHF
paired helical filaments
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- JNK
c-Jun NH2-terminal kinase
- GSK-3β
glycogen synthase kinase-3β
- Cdk5
cyclin-dependent kinase 5
- PKA
cAMP-dependent protein kinase
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- MBP
myelin basic protein
- GST
glutathione S-transferase
- PGSP-2
phosphoglycogen synthase peptide 2
References
- 1.Finch C, Tanzi R E. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini M G, Goedert M. Trends Neurosci. 1998;21:428–433. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Yung Y-C, Quinlan M, Wisniewski H, Binder L. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papasozomenos S C. Lab Invest. 1989;60:123–137. [PubMed] [Google Scholar]
- 5.Papasozomenos S C. Lab Invest. 1989;60:375–388. [PubMed] [Google Scholar]
- 6.Neve R L, Harris P, Kosik K S, Kurnit D M, Doulon T A. Mol Brain Res. 1986;1:271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M, Spillantini M G, Jakes R, Rutherford D, Crowther R A. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 8.Janke C, Beck M, Stahl T, Holzer M, Brauer K, Bigl V, Arendt T. Mol Brain Res. 1999;68:119–128. doi: 10.1016/s0169-328x(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 9.Himmler A. Mol Cell Biol. 1989;9:1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosik K S, Orecchio L D, Bakalis S, Neve R L. Neuron. 1989;2:1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee M K, Trojanowski J Q, Lee V M. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 12.Prohst A, Gotz J, Wiederhold K H, Tolnay M, Mistl C, Jaton A L, Hong M, Ishihara T, Lee V M-Y, Trojanowski J Q, et al. Acta Neuropathol. 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- 13.Papasozomenos S C, Autilio-Gambetti L, Gambetti P. J Cell Biol. 1981;91:866–871. doi: 10.1083/jcb.91.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binder L I, Frankfurter A, Rebhum L I. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papasozomenos S C, Binder L I. Cell Motil Cytoskeleton. 1987;8:210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Murphy M P, Baker M, Yu X, et al. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G V W, Jenkins S M. J Alzheimer's Dis. 1999;1:307–328. doi: 10.3233/jad-1999-14-511. [DOI] [PubMed] [Google Scholar]
- 18.Papasozomenos S C, Su Y. Proc Natl Acad Sci USA. 1991;88:4543–4547. doi: 10.1073/pnas.88.10.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papasozomenos S C. J Neurochem. 1996;66:1140–1149. doi: 10.1046/j.1471-4159.1996.66031140.x. [DOI] [PubMed] [Google Scholar]
- 20.Papasozomenos S C. Proc Natl Acad Sci USA. 1997;94:6612–6617. doi: 10.1073/pnas.94.13.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papasozomenos S C, Papasozomenos T. J Alzheimer's Dis. 1999;1:147–153. doi: 10.3233/jad-1999-1302. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Kondo J, Ihara Y. Science. 1987;235:1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 23.Hamos J E, Oblas B, Pulaski-Salo D, Welch W J, Bole D G, Drachman D A. Neurology. 1991;41:345–350. doi: 10.1212/wnl.41.3.345. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Roskoski R. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- 26.Szendrei G I, Lee V M-Y, Otvos L. J Neurosci Res. 1993;34:243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- 27.Lang E, Szendrei G I, Lee V M-Y, Otvos L. Biochem Biophys Res Commun. 1992;87:783–790. doi: 10.1016/0006-291x(92)91264-q. [DOI] [PubMed] [Google Scholar]
- 28.Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson G V W, Litersky J M, Schenk D, Lieberburg I, Trojanowski J Q, Lee V M-Y. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- 29.Goedert M, Jakes R, Crowther R A, Cohen P, Vanmechelen E, Vandermeeren M, Cras P. Biochem J. 1994;301:871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe N, Takio K, Hasegawa M, Arai T, Titani K, Ihara Y. J Neurochem. 1992;58:960–966. doi: 10.1111/j.1471-4159.1992.tb09349.x. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenberg-Kraag B, Mandelkow E-M, Biernat J, Steiner B, Schröter C, Gustke N, Meyer H E, Mandelkow E. Proc Natl Acad Sci USA. 1992;89:5384–5388. doi: 10.1073/pnas.89.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LoPresti P, Szuchet S, Papasozomenos S C, Zinkowski R P, Binder L I. Proc Natl Acad Sci USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmel G, Mager E M, Binder L I, Kuret J. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 34.Drewes G, Lichtenberg-Kraag B, Doring F, Mandelkow E M, Biernat J, Goris J, Doree M, Mandelkow E. EMBO J. 1992;11:2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latimer D A, Gallo J, Lovestone S, Miller C C, Reynolds C H, Marquardt B, Stabel S, Woodgett J R, Anderton B H. FEBS Lett. 1995;365:42–46. doi: 10.1016/0014-5793(95)00434-b. [DOI] [PubMed] [Google Scholar]
- 36.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 37.Adler V, Yin Z, Fuchs S Y, Benezra M, Rosario L, Tew K D, Pincus M R, Sardana M, Henderson C J, Wolf C R, et al. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takekawa P, Maeda T, Saito H. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merlin A B, Yaglom J A, Gabai V L, Mosser D D, Zon L, Sherman M Y. Mol Cell Biol. 1999;19:2547–2555. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P. FEBS Lett. 1997;409:57–62. doi: 10.1016/s0014-5793(97)00483-3. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds C H, Utton M A, Gibb G M, Yates A, Anderton B H. J Neurochem. 1997;68:1736–1744. doi: 10.1046/j.1471-4159.1997.68041736.x. [DOI] [PubMed] [Google Scholar]
- 42.Hong M, Lee V M-Y. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 43.Cohen P, Alessi D R, Cross D A E. FEBS Lett. 1997;410:3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- 44.Hetman M, Cavanaugh J E, Kimelman D, Xia Z. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere C A, Imahori K. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez A, Toro R, Cáceres A, Maccioni R B. FEBS Lett. 1999;459:421–426. doi: 10.1016/s0014-5793(99)01279-x. [DOI] [PubMed] [Google Scholar]
- 47.Patrick G N, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai L-H. Nature (London) 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 48.Imahori K, Uchida T. J Biochem. 1997;121:179–188. [PubMed] [Google Scholar]
- 49.Paudel H K, Lew J, Ali Z, Wang J H. J Biol Chem. 1993;268:23512–23518. [PubMed] [Google Scholar]
- 50.Jicha G A, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, Davies P. J Neurosci. 1999;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng-Fischhöfer Q, Biernat J, Mandelkow E-M, Illenberger S, Godemann R, Mandelkow E. Eur J Biochem. 1997;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- 52.Scott C W, Spreen R C, Herman J L, Chow F P, Davison M D, Young J, Caputo C B. J Biol Chem. 1993;268:1166–1173. [PubMed] [Google Scholar]
- 53.Litersky J M, Johnson G V W, Jakes R, Goedert M, Lee M, Seubert P. Biochem J. 1996;316:655–660. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soderling T, Derkach V A. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- 55.Strack S, Barban M A, Wadzinski B G, Colbran R J. J Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- 56.Liao H, Li Y, Brautigan D L, Gundersen G G. J Biol Chem. 1998;273:21901–21902. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- 57.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom G S, Mumby M C. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 58.Sontag E, Nenbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White C G, Mumby M C, Bloom G S. J Biol Chem. 1999;274:25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 59.Low B, Rossie S. J Biol Chem. 1995;270:12808–12813. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- 60.Chang Y, Abe A, Shayman J A. Proc Natl Acad Sci USA. 1995;92:12275–12279. doi: 10.1073/pnas.92.26.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans D B, Rank K B, Bhattacharya K, Thomsen D R, Gurney M D, Sharma S K. J Biol Chem. 2000;275:24977–24983. doi: 10.1074/jbc.M000808200. [DOI] [PubMed] [Google Scholar]
- 62.King M E, Ahuja V, Binder L I, Kuret J. Biochemistry. 1999;38:14851–14859. doi: 10.1021/bi9911839. [DOI] [PubMed] [Google Scholar]
- 63.Hall G F, Yao J, Lee G. Proc Natl Acad Sci USA. 1997;94:4733–4738. doi: 10.1073/pnas.94.9.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salbaum J M, Weidemann A, Lemaire H-G, Masters C L, Beyreuther K. EMBO J. 1988;7:2807–2813. doi: 10.1002/j.1460-2075.1988.tb03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shepherd C E, Bowes S, Parkinson D, Cambray-Deakin M, Pearson R C A. Neuroscience. 2000;99:371–325. doi: 10.1016/s0306-4522(00)00197-4. [DOI] [PubMed] [Google Scholar]