"We are in this business to serve people, and that means not only maintaining and restoring their health, but doing it without violating the budget they are willing to spend . . . The unifying principle is that when we run into a tough choice, we should try to resolve it by reference to the people we serve: what is important to them and how they weight the different consequences." David Eddy, MD, PhD.1
Evidence-based medicine (EBM) is a buzzword, part of a health care movement that claims to improve medical decision making, thus improving patient care1,2,3. The rationale for EBM is that medical literature is similar to legal evidence that is used to prove (or disprove) a belief4. The scientific framework of evidence-based medicine is 1) systematic reviews based on clinical trials and 2) validated outcome measurements 5 and 3) evidence is then used to guide clinical practice. How EBM will affect orthopaedic practice depends on the quality of evidence (numbers 1 and 2 above) provided by clinical research and the willingness of orthopaedists to adopt the "best evidence" into their delivery of care (number 3).
The goal of evidence-based clinical information is to provide scientific information to orthopaedists that translates into quality patient care while mindful of costs, ethics and safety. As many in the academic orthopaedic community have pointed out, there is a history of resistance to performing well-designed clinical trials of orthopaedic procedures6,7,8,9. Past neglect now leaves us with few published orthopaedic clinical trials, and the consequences are that orthopaedics lags behind many fields in the "raw material" for evidence based medicine. The next few years will be crucial for the production and application of orthopaedic evidence and momentum is gathering in our publications to provide more of that is needed as evidence.
For example, there are no clinical trials with modern outcome measures compare operative versus nonoperative treatment of large joint arthritis. This seems non-sensical to most orthopaedists. The principles of good orthopaedic practice are 1) first try non-operative treatment and 2) perform surgery when non-operative treatment fails. With this paradigm, there can be no valid comparison of operative and non-operative treatments because they are applied to mutually exclusive groups (though paradoxically the same patients!). What is needed in order to provide evidence is clinical trials that prove the efficacy of a surgical procedure versus other common treatments. And, in some instances there should be comparison of operative and non-operative care. That is how evidence will be assembled that answers patients' questions and provides the highest quality of orthopaedic care.
With the information explosion, patients have access to enormous amounts of information regarding diagnoses and treatments. I argue that the burden shifted to orthopaedic surgeons to provide evidence that they deliver the best available care to patients with musculoskeletal disorders. The case may be made that orthopaedic surgeons should insist on evidence-based guidelines for the highest quality, most cost-effective care for musculoskeletal patients4,11. The answer to the question of best orthopaedic treatment is to prove by patient-generated measurements that demonstrate how well orthopaedic surgery has improved the quality of life. In the case of fracture care, evidence may show that one treatment reduces impairment rather than provide an improvement in life quality.
Insurers and government payers currently monitor monetary cost, safety and durability (and sometimes the patient's return to work). In essence, they are holding orthopaedists fiscally accountable for professional behavior1, 5. What orthopaedic surgeons should add to financial accountability of patient care is patient satisfaction. Thus the case for orthopaedic EBM is going "one better" than the insurance industry or government accountability.
If patients prove that a hip replacement is better than medication and a cane, it is possible to justify expense and physical risks of surgery and the possible need for expensive revision surgery. If patients do not prove that a hip replacement is better than pills and a cane, then there is no need for further justificatio-it's a dead issue, no surgery. Once replacement arthroplasty for hip arthritis is justified, the same process justifies the application of improvements (changes) in technology. Thus if ceramic surface components are to prove more effective than the conventional metal-polyethylene interface, there must be patient oriented evidence to support that claim.
In a manufacturing analogy, EBM delivers information that is a high quality, cost-effective product to orthopaedic surgeon consumers. Basic research and product development produces information useful to clinical research that in-turn produce information that is packaged and transported as manuscripts and presentations at clinical conferences. Manuscripts that provide patient oriented evidence that matters (POEMs) are consumed by the orthopaedic surgeon and put into practice12. As defined, POEM is "patient-oriented evidence with which we can evaluate the efficacy of interventions in terms of results that patients care about and that we as clinicians want for our patients"13.
Feedback from practicing orthopaedists will generate the next round of basic research and product development in order to solve problems that arise from the first cycle of production (Figure 1). This is similar to the figure (Figure 2) illustrating production and marketing of gasoline from crude oil: raw material, refining, distribution, sales & marketing giving rise to the demand of more product. As a practical matter, EBM is the application (sales & marketing) of POEMs that are derived from valid clinical trials of orthopaedic procedures as published in the appropriate peer-reviewed journal (or presented at a peer-reviewed meeting). Clinical trials are the refinery that takes basic research or applied technology and generates useful information. The peer-reviewed journals (e.g., JBJS American- Evidence Based Orthopaedics), peer-reviewed Internet bulletin boards (ScHARR- www.shef.ac.uk/~scharr/ir/netting.html ) serve as the distribution network for the results of clinical trials and systematic reviews (Cochrane Database of Systemic Reviews, EBM Best Evidence).
Figure 1. Production Cycle for Orthopaedic EBM.

Production cycle for evidence-based orthopaedics.
Figure 2. Production Cycle for Gasoline.
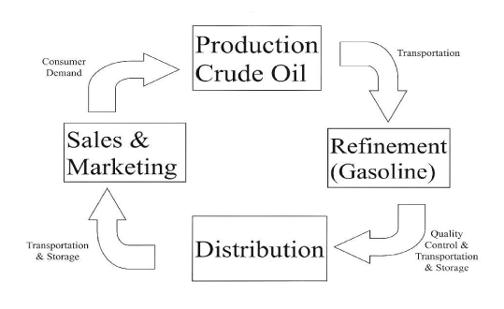
Production cycle for gasoline.
Several recent clinical trials in the English language orthopedic literature illustrate a trend toward providing the type of evidence needed to keep orthopaedics in the mainstream of health care 3,14,15,16,17,18. As this trend becomes more expansive and inclusive of musculoskeletal disorders, orthopaedic surgeons will more readily implement improvements and advancements in surgical treatment. This empowerment comes from the power of the people-patients want safe and effective treatments, at reasonable cost. Clinical trials and comparison studies are researches done on humans for the good of other people. When evidence from clinical trials pass the filter of scientific scrutiny and become POEM, it will benefit people when orthopaedists change their professional behavior by implementing the POEM.
Another example, if a relatively safe oral antibiotic given for a few days is as effective in reducing bone infections as a week of combination intravenous antimicrobials for open long bone fractures, this is worthy of implementation in practice. On the other hand, if ceramic hip prostheses are proven to produce far less particulate debris than conventional total hip, it still remains to be proven that painful loosening is diminished when using a ceramic device. What makes sense inferentially needs to be proven clinically, that is the reason for evidence in orthopaedic practice. Evidence based orthopaedics does not replace common sense and clinical judgment; it should reinforce good orthopaedic practice. And, orthopaedic surgeons still need to apply personal experience, a sense of risk, and a cost-benefit ratio to the individual patient. Because there is a guideline that suggests a treatment, does not mean that all patients get the same treatment.
The techniques and devices that are being developed for use in the future should prove their safety and efficacy in the court of best evidence. Similarly, evidence for pharmaceuticals commonly prescribed by orthopaedic surgeons should be held to the same standard as surgical technology. What evidence supports oral chondroitin sulfate/glucosamine compared with NSAIDs for painful osteoarthritis of the knee? Is there evidence for prescribing Cox-2 inhibitors versus less expensive anti-inflammatory medication?
As a profession, orthopaedic surgeons will learn how orthopaedic practice questions can be answered by valid clinical trials. Trials are expensive and take considerable time to produce results-research design; planning and execution are crucial to success. Though masking is difficult, there are several elements of randomized clinical trials that are relevant to orthopaedic surgery. Starting with a good question that can be answered by a clinical trial, the research team needs to design an ethical study that can capture enough patients to answer the question (Table 1).
TABLE 1. Characteristics of a Clinical Trial.
| Posing a good question | What patients are available for study? What treatments should be compared? What is the comparison of interest? What outcome shall be measured? Who will benefit from the results? |
| Deciding if the question is answerable/feasible | Can enough patients be enrolled? Statistical plan - likelihood of reaching significance? Affordable/fundable? Adequate surgeons with expertise? Are the surgeons able to provide different treatments without technical bias? Means of randomization - many cases are subverted by investigators' bias. |
| Ethical | Patients are free to decline or withdraw without coercion. Controls must be ethically decided (non-treatment is rarely ethical). Independent quality control/monitoring. Will the study give the best answer that will benefit patients? Financial incentives for patient enrollment, investigator? Full disclosure to insurers that won't pay for services as "part of a study"? Surgeons must have equipoise - i.e. no prejudice about treatment efficacy. |
Randomized clinical trials work much better for highly prevalent conditions than for rare events. It is easier to design a clinical trial for patients with low back pain and disc herniation than to determine the best surgery for adults with chondrosarcoma of the proximal humerus. Not all orthopaedic questions are answerable by clinical trials (e.g. risk factors in postoperative joint sepsis- this is a disease oriented measure) and other forms of patient oriented evidence are sometimes needed. Prospective clinical studies measuring outcomes that matter provide better evidence for decision making than disease oriented evidence 12,13.
These points are summarized, from the viewpoint of a clinical investigator by Cummings' acronym FINER: Feasible, Interesting to the investigator, Novel, Ethical and Relevant19. From the vantagepoint of the orthopaedic surgeon as consumer of evidence-based medicine, there is no authoritative orthopaedic resource that evaluates surgical clinical trials for accuracy and validity. But, efforts underway by the American Journal of Bone and Joint Surgery and orthopaedic subspecialty journals will soon produce a body of literature that will educate orthopaedic practitioners how to change with the times as good evidence is written.
This is not to say that orthopaedists have ignored good evidence in the past or refused to keep-up with improvements in patient care. What is new about EBM is the concept that orthopaedists change their practice more swiftly when compelling evidence is presented. The problem has been the lack of evidence and that is now being corrected. Orthopaedic surgeons who tried new things in the past have been "burned". With good reason, these surgeons are likely to resist moving quickly to implement a new procedure or technique. With the presence of one or two valid POEMs, evidence based orthopaedics decreases the likelihood of making bad practice decisions about using new technologies and procedures. Conversely, if a surgeon bases their decision to try a new technique based solely on safety and efficacy information (disease oriented evidence), there is greater probability that unreported problems or patient dissatisfaction will occur postoperatively. The occasional need to rely on entirely on disease oriented evidence is understandable if no POEMs exist.
As mentioned by Wright & Swiontkowski3,20,21, the number of published clinical trials in orthopaedic journals has dramatically increased in the past decade. The nature of randomized clinical trials is they require multiple centers and several surgeons and a large infrastructure, both personnel and electronic informatics. There are many logistical problems that include finding surgeons who are competent to provide different surgical treatments without bias, truly random allocation of patients to the treatment groups, enrolling adequate numbers of patients and keeping the enrollees participating for the duration of the study. How the principal investigator and the study designers eliminate or reduce bias is critical to the clinical trial. If there are serious (fatal) flaws in design or implementation, serious issues of bias or credibility, serious problems with safety, the result will not be accepted by the practicing orthopaedic surgeon-even if published and presented.
Statistical correctness6,7, akin to political correctness, is another issue that needs to be addressed in gaining the confidence of orthopaedists who do not have the training or inclination to understand the nuances of statistics and statistical terms. Evidence should be clearly recognized by reputable experts in the field and by local practitioners, without having an extensive knowledge of statistics. When there is no patient oriented evidence and orthopaedic practice is based upon disease oriented evidence, an orthopaedic research team should take up the challenge and design a clinical trial that measures treatment outcome in an attempt to make POEM. The creation and application of POEM is where a sea change in attitude is needed within the field of orthopaedic surgery.
As an aside, changing physician's behavior is not an easy task. A recent report by team of French epidemiologists indicated that when orthopaedists had access to a computer-based clinical decision support system, compliance increased with a validated deep vein thrombosis prophylaxis regimen22. And, when the computer system was removed from the hospital, surgeons reverted to their previous practice of haphazard DVT prophylaxis and the clinical outcome reflected the change in the surgeon's prescription. This type of study highlights the difficulty in changing surgeon's behavior, even when there clearly is benefit to do so.
Randomized clinical trials and evidence-based medicine for orthopaedic surgeons are not an oxymoron! Randomized clinical trials that answer orthopaedic questions, if given the chance, will provide the basis for much of the future practice of orthopaedic surgery. Orthopaedic literature is providing more and more evidence. The readers may not be completely familiar with the language of the investigator (e.g., statistics) but the conclusions (benefit to patients) should be clearly understandable-outcomes that are meaningful to patients. And, published orthopaedic POEM should be put into practice by orthopaedic surgeons for the benefit of their patients and to keep this process moving forward.
References
- 1.Eddy DM. Clinical Decision Making From Theory to Practice. Boston: Jones & Bartlett; 1996. pp. xi–xii. [Google Scholar]
- 2.Cook DJ, Mulrow CD, Haynes RB. In: Systematic reviews, synthesis of best evidence for health care decisions. Mulrow C, Cook D, editors. Philadelphia: American College of Physicians; 1998. pp. 5–12. [Google Scholar]
- 3.Wright JG, Swiontkowski MF. Introducing a new journal section: evidence-based orthopaedics. J Bone Joint Surg. (Am) 2000;82:759–760. [Google Scholar]
- 4.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence-based medicine: what it is and what it isn't. British Med J. 1996;312:71–71. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddy DM. The challenge. JAMA. 1990;263:287–290. [PubMed] [Google Scholar]
- 6.Colton C. Statistical correctness (editorial) J Orth Trauma. 2000;14:527–528. doi: 10.1097/00005131-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Matta JM. Striving for statistical significance: how important is it? J Orth Trauma. 2000;14:227–229. doi: 10.1097/00005131-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Laupacis A, Rorabeck CH, Bourne RB, Feeny D, Tugwell P, Sim DA. Randomized trials in orthopaedics: why, how and when. J Bone Joint Surg. (Am) 1989;71:535–543. [PubMed] [Google Scholar]
- 9.Rudicel S, Esdaile J. The randomized clinical trial in orthopaedics: obligation or option? J Bone Joint Surg. (Am) 1985;67:1284–1297. [PubMed] [Google Scholar]
- 10.Swiontkowski MF, Buckwalter JA, Keller RB, Haralson R. The outcomes movement in orthopaedic surgery: where we are and where we should go. J Bone Joint Surg. (Am) 1999;81:732–740. doi: 10.2106/00004623-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Sheldon TA, Guyatt GH, Haines A. Getting research into practice. When to act on the evidence. British Med J. 1998;317:139–142. doi: 10.1136/bmj.317.7151.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slawson D, Shaughnessy A. Are we providing doctors with the training and tools for lifelong learning? British Med J. 1999;319:1–3. [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz SR, Slawson D, Shaughnessy A. Orthopaedic information mastery: applying evidence-based information tools to improve patient outcomes while saving orthopaedists' time. J Bone Joint Surg. (Am) 2000;82:888–893. doi: 10.2106/00004623-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari M, Morrow F, Kulkarni AV, Tornetta P., III Meta-analysis in orthopaedic surgery. A systemic review of their methodologies. J Bone Joint Surg. (Am) 2001;83:15–24. [PubMed] [Google Scholar]
- 15.Patzakis MJ, Bains RS, Lee J, Shepherd L, Singer G, Ressler R, Harvey P, Holton P. Prospective, randomized, double-blind study comparing single-agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. J Orth Trauma. 2000;14:529–533. doi: 10.1097/00005131-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Pijnenburg ACM, Van Dijk CN, Bossuyt PMM, Marti RK. Treatment of ruptures of the lateral ankle ligaments: a meta-analysis. J Bone Joint Surg. (Am) 2000;83:761–773. doi: 10.2106/00004623-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Swiontkowski MF, Hanel DP, Vedder NB, Schwarrach JR. A comparison of short and long-term intravenous antibiotic therapy in the postoperative management of adult osteomyelitis. J Bone Joint Surg British. 1999;81:1046–1050. doi: 10.1302/0301-620x.81b6.9794. [DOI] [PubMed] [Google Scholar]
- 18.Swiontkowski MF, Agel J, McAndrew MP, Burgess AR, MacKenzie EJ. Outcome validation of the AO/OTA fracture classification system. J Orth Trauma. 2000;14:524–541. doi: 10.1097/00005131-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Cummings SR, Browner WS, Hulley SB. Conceiving the research question. In: Hulley SB, Cummings SR, editors. Designing Clinical Research. Baltimore: Williams & Wilkins; 1988. pp. 12–17. [Google Scholar]
- 20.Swiontkowski MF. The role of the orthopaedic surgeon as clinical site investigator. In: Weinstein JN, editor. Orthopaedic Clinical Trials: design, implementation, and regulation issues. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000. pp. 67–69. [Google Scholar]
- 21.Wright JG. Types of clinical research focusing on surgical trials. In: Weinstein JN, editor. Orthopaedic Clinical Trials: design, implementation, and regulation issues. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000. pp. 9–13. [Google Scholar]
- 22.Durieux P, Remy N, Ravaud P, Mounier N, Lepage E. A clinical decision support system for prevention of venous thromboembolism: effect of physician behavior. JAMA. 2000;283:2816–2821. doi: 10.1001/jama.283.21.2816. [DOI] [PubMed] [Google Scholar]


