Abstract
Background:
The natural history of cervical spondylotic myelopathy is frequently one of slow, progressive neurological deterioration. The operative treatment for patients with moderate to severe involvement is decompression of the spinal cord. Laminectomy has been a traditional approach and laminoplasty has developed as an attractive alternative. The purpose of this study was to examine and compare the outcomes of these two procedures in similar groups of patients at a five year average follow-up.
Methods:
A consecutive series of twenty patients who underwent open-door laminoplasty for multi-level cervical spondylotic myelopathy or radiculopathy was compared to a similar group of 22 matched patients who underwent multi-level laminectomies. Patients were similar in age, gender, number of operative levels, and length of follow-up. At the latest examination, each patient underwent a comprehensive neurological evaluation. A modification of the Nurick classification was used to assess the degree of myelopathy. Radiographs at latest follow-up were assessed for instability, and measurements of the space-available-for-the-cord and Pavlov ratio were made at involved levels.
Results:
Myelopathy, as determined by our modified Nurick scale, improved from a preoperative average of 2.44 to 1.48 in laminoplasty patients and from an average of 3.09 to 2.50 in laminectomy patients. Pain improved 57 percent and 8 percent in laminoplasty and laminectomy groups, respectively. Subjective neck stiffness was not significantly different based on the numbers available, although laminoplasty patients demonstrated some loss of range of motion on examination. The only variable that predicted the postoperative degree of myelopathy in both groups was the preoperative degree of myelopathy.
Conclusions:
Laminectomy and laminoplasty patients demonstrated improvements in gait, strength, sensation, pain, and degree of myelopathy. Laminoplasty was associated with fewer late complications. Based on this analysis, we believe that laminoplasty is an effective alternative to laminectomy in patients with multi-level cervical spondylotic myelopathy or radiculopathy.
INTRODUCTION
Several procedures are available for the operative management of multilevel cervical spondylotic myelopathy or radiculopathy.16 Laminectomy has proven to be successful,6,22,36 but several inherent risks have been recognized. Postoperative instability and deformity, in particular kyphosis, is a well-documented problem.2,4,5,17,23 Development of postoperative hematoma and peridural scar formation has also been documented. Expansive open-door laminoplasty, introduced by Hirabayashi in 1977,18,20 decompresses the spinal cord with two theoretical advantages: 1) preservation of some posterior elements minimizes the possibility of postoperative instability or deformity while expanding the room available for the cord; 2) it may limit the formation of hematoma and postoperative membranes.
The purpose of this study was to examine and compare the outcomes of these two procedures in similar groups of patients at a five year average follow-up. In addition, we wished to examine preoperative variables and determine their association, if any, with postoperative recovery. We are aware of no previously published study of this length follow-up that excludes the diagnosis of ossification of the posterior longitudinal ligament (OPLL) and compares laminectomy and laminoplasty. Our hypothesis is that both of these procedures provide adequate decompression of the spinal cord, however, laminoplasty may avoid the postoperative problem of instability.
MATERIALS AND METHODS
A consecutive series of twenty patients who underwent open-door laminoplasty by a single surgeon (CRC) for multi-level cervical spondylotic myelopathy or radiculopathy was compared to a similar group of 22 patients who underwent multi-level laminectomies. Each patient had a minimum three-year follow-up with an average of approximately five years (range 36 to 112 months). Laminectomy patients were matched in terms of age, length of follow-up, gender, and number of operative levels.
The selection of the procedure was at the discretion of the individual surgeon. All patients but one from the laminoplasty group had recent radiographic follow-up.
Indications for the procedures included patients with cervical spondylotic myelopathy or myeloradiculopathy who had progressive neurological symptoms, bowel or bladder alterations, failure of non-operative management, and multilevel (greater than two) involvement. Patients with ossification of the posterior longitudinal ligament, post-traumatic injuries, tumorous conditions, and underlying instability were excluded.
The two groups were similar in age, length of follow-up, number of involved levels, and gender (Table 1). The average age of the patients in the laminoplasty group was 53.5 years (range 41 to 75 years), and the average age in the laminectomy group was 54.3 years (range 33 to 73 years). Follow-up averaged 65.4 months (range 36 to 112 months) in the laminoplasty group, and 64.8 months (range 53 to 76 months) in the laminectomy group. An average of 4.3 levels (range 3 to 6 levels) were involved in the laminoplasty group and 4.6 levels (range 3 to 5 levels) in the laminectomy group. Two of twenty patients in the laminoplasty group, and three of 22 patients in the laminectomy group were women. Five patients had prior neck operations in the laminoplasty group, and one patient in the laminectomy group. Among laminoplasty patients, one had a three-level procedure, 12 had four-level procedures, and 7 had five-level procedures. Three laminectomy patients had three-level procedures, 7 had four-level procedures, 7 had five-level procedures, and 5 had six-level procedures. Cervical 2 was an operative levels in 8 laminectomy patients and in none of the laminoplasty patients.
TABLE 1. Preoperative Comparison of Laminoplasty and Laminectomy Patients.
| Laminoplasty | Laminectomy | |
|---|---|---|
| Age (yrs.) | 53.5 (41-75) | 54.3 (33-73) |
| Length of Follow-up (mos.) | 65.4 (36-112) | 64.8 (53-76) |
| Female/Total | 2/20 | 3/22 |
| Avg. # Operative Levels | 4.3 (3-5) | 4.6 (3-6) |
| SAC (mm) | 14.1 (10-17) | 15.2 (13-18) |
| Pavlov ratio | .64 (.46-.75) | .71 (.54-1.09) |
SAC= Space Available for the Cord
At latest follow-up, each patient underwent a complete evaluation including neurological examination. The examination was performed by a surgeon (SBK) not involved with the operative procedures. One laminoplasty patient had his most recent follow-up at an outside facility. All patients had had preoperative cervical anteroposterior and lateral flexion and extension cervical spine radiographs and either magnetic resonance imaging or computer tomography scans with myelography, or both. The evaluation included the location and degree of preoperative and postoperative pain using an analog scale, subjective and objective alteration in sensation and weakness, presence of hyperreflexia, clonus, Babinski sign, Hoffman sign, walking difficulty, bowel or bladder changes, neck stiffness, cervical range-of-motion, use of pain medication postoperatively, occupation, smoking habits, prior neck operations, and pertinent medical history. The evaluation also included assessment of the degree of myelopathy based on a modification of the Nurick classification38 (Table 2). An additional category, no cord or root symptoms, was added. The Prolo anatomic-economic-functional score was calculated for each patient (Table 3).40
TABLE 2. Modified Nurick Classification39 .
| Grade 0 | No root or cord symptoms |
| Grade I | Root signs or symptoms. No evidence of cord involvement. |
| Grade II | Signs of cord involvement. Normal gait. |
| Grade III | Mild gait abnormality. Able to be employed |
| Grade IV | Gait abnormality prevents employment. |
| Grade V | Able to ambulate only with assistance. |
| Grade VI | Chair bound or bedridden |
TABLE 3. Prolo Anatomic-Economic-Functional Rating System (1-10)41 .
| Economic status | |
|---|---|
| E1 | Complete invalid |
| E2 | No gainful occupation (including ability to do housework or continue retirement activities) |
| E3 | Able to work but not at previous occupation |
| E4 | Working at previous occupation with no restrictions of any kind |
| E5 | Able to work at previous occupation with no restrictions of any kind |
| Functional Status | |
| F1 | Total incapacity (or worse than before operation) |
| F2 | Mild to moderate level of pain (or pain same as before operation but able to perform all daily tasks of living) |
| F3 | Low level of pain and able to perform all activities except sports |
| F4 | No pain, but patient has had one or more recurrences of neck or radicular pain |
| F5 | Complete recovery, no recurrent episodes of pain, able to perform all previous activities |
The preoperative space-available-for-the-cord (SAC) was determined for both laminoplasty and laminectomy patients. To control for variation in radiograph magnification, the Pavlov ratio (comparison of anteroposterior canal diameter to the anterior-posterior vertebral body diameter in the sagittal plane) was also calculated.46 The postoperative space-available-for-the-cord and Pavlov ratios were determined for laminoplasty patients only since it was not possible to determine these values in the laminectomy patients due. Evidence of pre- and postoperative deformity or instability was evaluated on postoperative dynamic films. Radiographic analysis was then repeated to assess intra-observer reliability.
In the laminoplasty group, preoperative pain was solely in the neck, shoulders, or both in 3 patients, in the neck and extremities in 10, and in extremities alone in 7. Five patients had neck or shoulder pain only preoperatively in the laminectomy group, 4 in the neck and extremities, and 5 in extremities alone, and 9 had no or minimal discomfort. On a visual analog scale of 1 to 10, from least to most, the average pain level was 7.7 in the laminoplasty patients and 4.7 in the laminectomy patients. This difference was significant (p=.018).
Neck stiffness was subjectively graded on a three point scale as follows: 1- no or occasional neck stiffness, 2- intermittent or frequent neck stiffness, but no difficulties with activities of daily living, and 3- neck stiffness causing difficulty or interfering with activities of daily living (ADL's). The average score preoperatively was 1.73 and 1.68 in the laminoplasty and laminectomy groups, respectively.
Preoperatively, 16 of 20 laminoplasty patients described subjective sensory changes including numbness or tingling in the upper extremities, lower extremities, or both. Seventeen of 22 laminectomy patients described similar sensory changes. Sixteen laminoplasty patients believed that weakness in the upper extremities, lower extremities, or both was a problem, and 17 laminectomy patients also believed weakness was a problem. Sixteen laminoplasty patients and 17 laminectomy patients described gait changes that affected their ability to walk, or the distance they walked, or required them to use some support or walking aide. Two laminoplasty patients and 6 laminectomy patients described the acute onset of bowel changes, bladder changes, or both.
Preoperative physical examination revealed 11 laminoplasty patients with sensory changes, and 16 laminectomy patients with alterations in sensation. Objective motor weakness in any muscle group of the upper or lower extremities during manual motor testing was identified in 13 laminoplasty patients and in 14 laminectomy patients. Gait abnormalities were observed in 11 laminoplasty and 16 laminectomy patients. Hyperreflexia was demonstrated in 13 laminoplasty and 18 laminectomy patients. A Hoffman sign was elicited in 11 laminoplasty patients, clonus in 8, and Babinski in 6. Nine laminectomy patients had a Hoffman sign, 9 had clonus of more than 2 beats, and the presence of a Babinski sign was seen in 8.
Using the modified Nurick classification, the average preoperative score for laminoplasty patients was 2.44 (range 1 to 5) and 3.09 (range 1 to 6) for laminectomy patients (p<.0001). Eighteen laminoplasty patients were diagnosed with myelopathy or myeloradiculopathy, and 2 had radiculopathy only. 19 laminectomy patients were diagnosed with myelopathy or myeloradiculopathy and 3 with radiculopathy alone.
Nine laminectomy patients were working preoperatively in jobs requiring moderate to heavy labor (for example, mechanic, carpet layer, or construction work). One was unemployed, 3 were retired, and 2 were disabled. Six laminoplasty patients were involved in jobs requiring moderate to heavy labor. One was unemployed, one retired, and 5 disabled. Smoking pack-years averaged 51 in laminoplasty patients and 33 in laminectomy patients.
Preoperative radiographs demonstrated an average space-available-for-the-cord at the most involved level of 14.1 millimeters (range 10 to 17 millimeters) in the laminoplasty group. The average space-available-for-the-cord for all involved levels was 15.2 millimeters (range 11.8 to 17 millimeters) and the Pavlov ratio averaged .64 (range .46 to .75) preoperatively in the laminoplasty group. The space available for the cord averaged 15.2 millimeters (range 13 to 18 millimeters) at the narrowest level and 16.5 millimeters (range 13.7 to 19.6 millimeters) at all operative levels in laminectomy patients. The Pavlov ratio averaged .71 (range .54 to 1.09) preoperatively in laminectomy patients.
OPERATIVE TECHNIQUE OF LAMINOPLASTY
A standard midline posterior approach is performed, and the posterior elements are exposed. The interspinous and supraspinous ligaments are left intact. Decompression begins at the least involved levels and then proceeds to the more stenotic levels. A cutting burr is then used to make a gutter through the outer cortex of the bone at the lamina-facet junction. A diamond burr then deepens the gutter through the inner cortex. The opened side is selected according to the most symptomatic side. A channel through the outer table only on the hinged side is made next with the cutting burr. The supraspinous and interspinous ligaments and the ligamentum flavum are transected at the levels immediately above and immediately below the laminoplasty. A thin Kerrison ronguer is used to complete the trough, and dural adhesions from the undersurface of the lamina are gently removed. The so-called laminar door is opened slowly to prevent excessive traction on spinal nerve roots (Figure 1). Foraminotomies are performed as needed in patients with radiculopathy. When rib allograft is used, a segment is cut into appropriate sized wedges and a trough made on either end with the cutting burr. This is then fitted between the articular processes and the edges of the lamina, propping the laminar door open (Figure 2). A hard collar is used for the first eight weeks after the operation, followed by a soft collar for an additional 4 to 8 weeks.
Figures 1-A and 1-B. Pre-and postoperative radiographs of a 65 year-old man with progressive myelopathy at the time of laminoplasty.
Fig. 1-A. Preoperative lateral radiograph demonstrating notable canal stenosis.
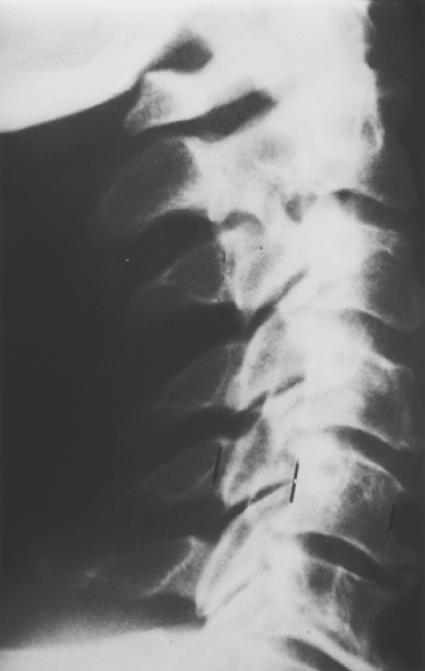
Fig. 1-B. Postoperative lateral radiograph showing a 7 millimeter expansion of the space-available-for-the-cord following laminoplasty using Hirabyashi's open-door technique.
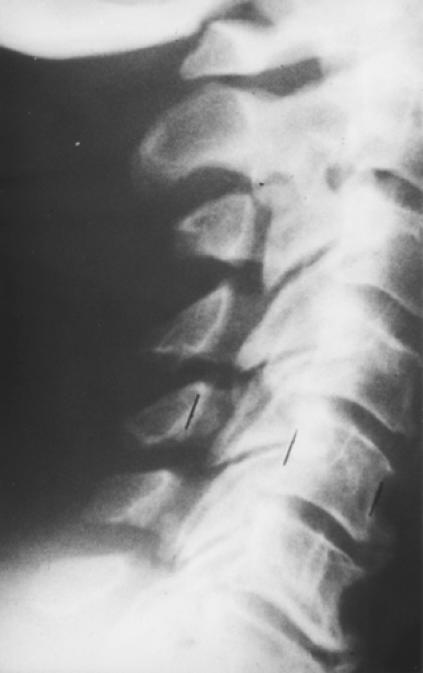
Figure 2. Postoperative computer tomography scan of laminoplasty patient using rib allograft supplementation.
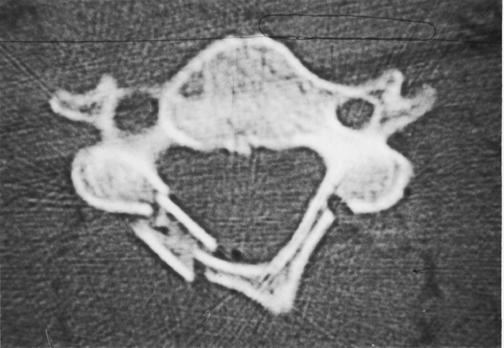
Note notching of rib allograft to secure laminar "door" and burred channel on hinged side through outer table only.
In the laminoplasty group, the first 10 patients underwent the standard Hirabayashi open-door technique.20 In the next ten patients rib allograft was used to secure the opening of the laminoplasty. In the standard procedure, stay sutures, which are placed to prevent the door from closing, are placed through the deep muscles and capsules around the facet joints of the hinged side. The stay sutures are tied to the spinous processes through the ligamentum flavum to keep the laminar door open. In the modified technique, a segment of rib allograft is obtained and a trough burred at both ends. The grooved allograft is then fitted into the opening, hinging the open-door. Laminectomies were performed in standard fashion. The amount of facet resection, if any, was dictated by the abnormal findings on preoperative neurodiagnostic studies and at operation. Intraoperative monitoring of the spinal cord was used with few exceptions.
STATISTICAL ANALYSIS
The relationship between preoperative variables including age, smoking, space-available-for-the-cord, Pavlov ratio, and degree of myelopathy and outcome variables of postoperative modified Nurick classification, residual pain, Prolo score, postoperative weakness, sensation, and gait changes were examined. Postoperative space-available-for-the-cord and Pavlov ratio in laminoplasty patients was analyzed to see if they correlated with these postoperative variables. Multiple regression analysis, Fisher's exact test, Wilcoxon sign-rank test, Wilcoxon two-sample test, paired t-test, Pearson, and Spearman coefficients were among the statistical tools used.
RESULTS
Operative time averaged 201 minutes for the laminoplasty procedures and 165 minutes for laminectomy procedures. Blood loss averaged 505 milliliters (range 100 to 1500 milliliters) in laminoplasty cases and 310 milliliters (range 40 to 1450 milliliters) in laminectomy procedures.
Based on the numbers available, neither the preoperative space-available-for-the-cord at the narrowest sagittal dimension, average space-available-for-the-cord of all involved levels, nor the preoperative Pavlov ratio predicted or correlated with the outcome variables of degree of myelopathy using the postoperative modified Nurick classification, residual pain, Prolo score, or postoperative weakness, sensation, and gait changes in both laminoplasty or laminectomy groups. Age had no statistical correlation with these outcome parameters, and smoking habits as determined by number of pack-years had no relationship either.
Overall, the level of pain postoperatively correlated with the level of pain preoperatively (P<.001, r=.71). However, when the individual groups were analyzed this correlation was present only for the laminectomy group and not the laminoplasty group. Laminectomy patients with a higher degree of pain preoperatively were more likely to have a higher degree of pain postoperatively.
The only variable that predicted the degree of myelopathy postoperatively in both groups, as measured on the Nurick scale, was the degree of myelopathy preoperatively. Those with a lesser degree of myelopathy or lower Nurick scores had better outcomes than those patients with advanced myelopathy and higher Nurick grade (r=0.74 and 0.84, p<.0001, in laminoplasty and laminectomy patients, respectively).
Among laminoplasty patients, a higher postoperative space-available-for-the-cord and Pavlov ratio were associated with an improved level of motor strength (20 millimeters versus 16.8 millimeters, p=.04). The postoperative space-available-for-the-cord was not associated with postoperative Nurick grade, postoperative pain, Prolo score, sensory improvements, or objective findings of clonus, Hoffman sign, and Babinski sign.
The postoperative Pavlov ratio was related to muscle strength, gait, and postoperative Nurick grade. Those with improvement in motor grades had an average Pavlov ratio of .90 versus 0.68 for those that did not improve (p=.009). Those patients with improved or normalized gait had an average Pavlov ratio of .90 versus 0.68 with those with continued gait difficulties (p=.0072). The postoperative Pavlov ratio was inversely correlated to Nurick grade ( r=.-49, p=.036). Intraobserver reliability demonstrated a high level of agreement and minimal variability in evaluation of space-available-for-the-cord and Pavlov ratios.
Postoperative improvement in outcome variables are detailed in Table 4. Seven laminoplasty patients and 7 laminectomy patients had objective improvements in sensory deficits. Partial or complete recovery from motor weakness occurred in 12 laminoplasty patients and in 9 laminectomy patients. Gait improvement was seen in 7 laminoplasty patients and in 7 laminectomy patients. Bowel or bladder symptoms improved in all 6 of the laminoplasty patients with preoperative symptoms and in 4 laminectomy patients. Hyperreflexia, Hoffman sign and clonus resolved in 6, 7, and 3 laminoplasty patients, respectively, and resolution of hyperreflexia and clonus occurred in 3 and 2 laminectomy patients, respectively. At the latest postoperative examination, a Hoffman sign was a new clinical sign in two laminectomy patients. Subjectively, 10 laminoplasty patients and 7 laminectomy patients perceived improvements in sensation. Ten laminoplasty patients and 9 laminectomy patients had subjective improvement in motor strength. With the numbers available, these differences did not reach statistical significance.
TABLE 4. Postoperative Improvement in Outcome Variables.
| Laminoplasty | Laminectomy | |
|---|---|---|
| Objective weakness | 12/13 | 7/14 |
| Objective sensation | 7/11 | 7/16 |
| Subjective weakness | 10/17 | 9/18 |
| Subjective sensation | 10/20 | 7/17 |
| Gait | 7/11 | 7/16 |
| Bowel/bladder | 2/2 | 4/6 |
| Hyperreflexia | 7/11 | 3/18 |
| Hoffman sign | 7/11 | 2/22* |
| Clonus | 3/8 | 2/9 |
| Babinski | 1/6 | 3/22* |
New clinical findings in two patients identified during the latest postoperative examination
Pain scores improved 57 percent to an average of 3.2 in the laminoplasty patients and improved 8 percent in laminectomy patients to an average of 4.4 (p=0.0036). At latest follow-up, 10 laminoplasty patients were taking pain medications, either acetaminophen or an nonsteroidal anti-inflammatory, on a regular basis for pain. Thirteen laminectomy patients were taking pain medications. Four of them were taking narcotic medications, and the remainder taking acetaminophen or nonsteroidal anti-inflammatories. Five laminoplasty patients had neck pain only, 4 with neck and extremity pain, and 4 with extremity pain only. Four laminectomy patients had neck pain only, 3 with neck and extremity pain, and 7 with extremity pain only.
Neck stiffness scores increased to an average of 2.0 in the laminoplasty group and 1.9 in the laminectomy group, from an average of 1.7 preoperatively in both groups. This difference was not statistically significant.
Myelopathy, as graded with the modified Nurick scale improved 44 percent among laminoplasty patients to an average of 1.48 and 18 percent in laminectomy patients to an average of 2.50. This difference was significant (p<.0001). (Table 5).
TABLE 5. Changes in Pain, Modified Nurick Grade, and Neck Stiffness.
| Laminoplasty | Laminectomy | p value | |
|---|---|---|---|
| Pain level Pre-op (1-10) | 7.7 | 4.7 | .018 |
| Pain level Post-op (1-10) | 3.2 | 4.4 | .14 |
| Pain % Change | -57% | -8.0% | .0036 |
| Modified Nurick Pre-op | 2.44 | 3.09 | <.0001 |
| Modified Nurick Post-op | 1.48 | 2.50 | <.0001 |
| Modified Nurick % Change | -43.6% | -17.8% | <.0001 |
| Neck Stiffness Pre-op (1-3) | 1.7 | 1.7 | .76 |
| Neck Stiffness Post-op (1-3) | 2.0 | 1.9 | .365 |
The Prolo scores averaged 7.2 in each group. Ten laminoplasty patients had good or excellent results (Prolo score of 8-10) and 10 laminectomy patients had good or excellent results. Postoperatively, one patient was involved in moderate to heavy labor among laminoplasty patients. Nine were disabled and 4 were retired. Four laminectomy patients had occupations requiring moderate to heavy labor. Seven patients were disabled and 5 retired.
Postoperative radiographs revealed an average space-available-for-the-cord of 19.3 millimeters (14 to 24 millimeters) in laminoplasty patients at the narrowest level in the spinal cord. This sagittal diameter improved an average 5.2 millimeters or 27 percent (p<.0001). The Pavlov ratio improved to an average of 0.85 (range .58-1.21) or 33 percent (p<.0001). Range of motion for laminoplasty patients averaged 27 degrees in extension and 42 degrees in flexion. This compares to 43 degrees in extension for laminectomy patients (p=.0002) and 45 degrees of flexion. Right and left bending averaged 34 degrees and 45 degrees for laminoplasty and laminectomy groups respectively (p=.009). Right and left rotation averaged 48 and 58 degrees in laminoplasty and laminectomy patients respectively (p=.069).
COMPLICATIONS
Three complications occurred in the laminoplasty group. One patient had closure of the open door. He had been treated with the original Hirabayashi technique of suturing the laminar door open. Two patients had transient C5 paresis occurring one and five days after the operation. Both patients had eventual resolution of this new weakness- one by 11 months and one by 32 months.
One early and 5 late complications occurred in the laminectomy group (Fig. 2). One patient had a wound dehiscence that was treated with an irrigation and debridement and secondary healing. Five patients demonstrated radiographic signs of instability as defined by Panjabi and White48. Two patients had C 4/5 subluxation of 4-5 millimeters. These two also had a kyphosis measuring 19 and 33 degrees on dynamic films. Three other patients also demonstrated a kyphosis measuring 28, 31, and 38 degrees. One of these patients eventually underwent anterior decompression and arthrodesis.
DISCUSSION
Cervical spondylotic myelopathy and radiculopathy is often a progressively debilitating condition, and operative intervention is frequently warranted to alter the natural history.6,7,9,12,33,38,44 Anterior and posterior procedures have been developed to halt further deterioration and ameliorate present symptoms. Anterior decompression, corpectomy and arthrodesis is an excellent treatment alternative, but not without substantial complications.1,8,10,19,49 The anterior approach may be particularly difficult in patients with multi-level involvement and underlying congenital or developmental stenosis. However, the anterior approach is the senior author's (CRC) preferred approach for the operative management of the majority of patients with cervical spondylotic myelopathy and radiculopathy.
The posterior approach offers simplicity, less potential operative risk, and allows decompression away from the offending abnormalities including osteophytes, ossified or buckling ligaments, and disc protrusions. Laminectomy is the most commonly performed operation, but laminoplasty is gaining acceptance. Both procedures allow for dorsal cord migration, decreasing axial tension and improving vascular perfusion. Late complications, in particular post-laminectomy kyphosis or instability in multi-level procedures, are well-known complications, although the prevalence varies. Open door laminoplasty, introduced by Hirabayashi, attempts to avoid these complications . Decompression of the spinal cord and preservation of supporting structures could minimize the potential for iatrogenic instability and expand the sagittal dimension of the spinal canal. This procedure is not without criticism. Axial pain, limitation of range-of-motion, and limited long-term data are among the areas of concern.
The two groups of patients, laminectomy and laminoplasty, were similar with respect to age, length of follow-up, gender ratio, and number of operative levels. With the numbers available, no statistically significant difference could be demonstrated between the groups preoperatively with respect to the number of patients with subjective or objective alterations in sensation or weakness, gait disturbance, hyperreflexia, Hoffman sign, clonus and presence of a Babinski sign. Several differences, however, are with noting. Although the space-available-for-the-cord on preoperative radiographs was, with the numbers available, not significantly different (15.2 millimeters versus 16.5 millimeters), the Pavlov ratio was (.64 versus 0.71). Of importance was the degree of myelopathy at the time of operation. Using our modified scale of Nurick grading, the laminectomy group demonstrated significantly worse degree of myelopathy preoperatively (3.1 vs. 2.4, p<.0001). The higher Nurick grades among laminectomy patients may have indicated a fixed component of myelopathy and led to a poorer outcome.
Although laminectomy patients were worse at presentation based on myelopathy classification, laminoplasty patients, who were worse by radiographic criteria preoperatively had better improvement of their neurological signs. The average Nurick score improved almost by a full grade, from 2.44 to 1.48 among laminoplasty patients, while laminectomy patients improved approximately one-half a Nurick grade from 3.09 to 2.5 (p<.0001).
Although the postoperative Pavlov ratio did demonstrate a significant correlation with the final Nurick grade, this association was weak (p=.036, r=-.49). The only factor that predicted the postoperative degree of myelopathy in both groups was the preoperative degree of myelopathy as designated by the Nurick grade (p<.0001, r=.74 for laminectomy group, p<.0001, r=.84 in laminoplasty group). In addition, a Pavlov ratio of 0.94 and 0.91 or greater correlated with improvements in gait and muscle strength, respectively. Conversely, patients with a Pavlov ratio of less than 0.74 and 0.68 did not demonstrate objective improvements in gait and motor strength, respectively. A space-available-for-the-cord of 20 millimeters or more postoperatively was associated with improvements in motor grades in laminoplasty patients.
Clinical signs of myelopathy such as gait abnormality, Hoffman sign, clonus, and presence of a Babinski sign resolved more often in laminoplasty patients. Subjective and objective symptoms and signs of weakness and sensory deficits also demonstrated more substantial improvements in laminoplasty patients. With these small numbers, however, statistical significance could not be determined.
Initial average pain scores of 7.7 and 4.7 in laminoplasty and laminectomy groups, respectively, improved to 3.2 and 4.4 for 57 percent and 8 percent improvements, respectively. Thus, although laminoplasty patients had worse pain symptoms preoperatively, they demonstrated more substantial improvements and a lower overall level of pain postoperatively. Additionally, the strongest predictor of pain postoperatively among laminectomy patients was the preoperative level of pain. There was not a similar association in the laminoplasty group.
Although the Nurick grade, pain symptoms, and signs of myelopathy such as weakness, sensation, and gait change improved more among laminoplasty patients, the Prolo economic-functional outcome scores demonstrated similar results with 10 of 20 patients and 10 of 22 patients with good or excellent outcome in the laminoplasty and laminectomy groups, respectively. We believe these outcomes are due to the high proportion of individuals either retired or disabled in both groups (laminoplasty, 13 of 20 and laminectomy, 12 of 22), dramatically lowering the Prolo scores. Those disabled were more likely to be older and in labor-intensive jobs.
Neck stiffness has been frequently cited as a postoperative problem in laminoplasty. Hirabayashi and Yoshida found a limitation of approximately 50 percent in range-of-motion.19,52 Thus, we were not surprised to find that neck range-of-motion was limited in laminoplasty patients in our series. Extension and bending were affected the most (p=.0002 and p=.009, respectively, compared to laminectomy), although there were also reductions in flexion and rotation. The degree of limitation of activities, however, was not found to be functionally limiting and subjectively neck stiffness was not a frequently cited problem. Only one patient believed that neck stiffness interfered with his daily activities.
Neck stiffness following laminoplasty may serve a protective function. The stability of the spine following laminoplasty has been examined in biomechanical studies comparing cervical laminoplasty with laminectomy.38,51 Nowinski et al. examined nine cadaveric specimens after multilevel laminoplasty or laminectomy had been performed.37 Cervical spine levels 2 to 7 were tested with physiologic loading. Cervical laminectomy with 25 percent or more facetectomy resulted in highly significant increase in cervical motion for all motions compared to the intact controls. With the numbers available, cervical laminoplasty was not significantly different from the intact control, except for a marginal increase in axial torsion. This limitation of mobility can prevent late instability and neurological deterioration.
Neck and shoulder pain have been reported to occur at a higher rate postoperatively in laminoplasty patients. The difference in axial symptoms between our groups, however, was not significant.
Transient neurological worsening occurred in 2 laminoplasty patients and none of the laminectomy patients. Cervical 5 and 6 neuropraxia occurred in these two patients. One patient demonstrated complete recovery within 11 months, and another by 32 months. This phenomenon of post operative nerve root palsy occurring within several days of the operation is a well-documented problem. Cervical 5 and 6 appear to be the most commonly affected, and the injury usually motor-dominant. In Hirabayashi's series of 90 laminoplasties, seven patients had transient weakness in cervical 5 and 6 motor elements- four on the open side and three on the hinged side.20 Transient paresis in the cervical 5 distribution, cervical 6 distribution, or both has also been noted by Yoshida (3 of 40 cases)52, Yonenobu (3 of 42)51, and O'Brien's (1 of 10).39 The cause of this problem has not been elucidated, although tethering of the nerve roots with posterior migration of the cord has been suggested.51 We slowly elevate the laminar door during the laminoplasty in order to avoid excessive traction of the nerve roots as recommended by Aita et al.1
Post-laminectomy instability or kyphosis occurred with alarming frequency among laminectomy patients. One required an operation and two were planning for operative intervention. The prevalence of post-laminectomy kyphosis, a challenging problem to treat because of the lack of posterior elements, varies in the literature.25,27,28,43,53 No instances of instability occurred among laminoplasty patients. The etiology of post-laminectomy deformity is primarily mechanical, from loss of posterior support. As little as 25 percent facetectomy significantly increases motion in all directions37, and 50 percent facetectomy allows visualization of 3-5 millimeters of the nerve root.41 The amount of facet resection could have played a critical determinant in those cases with instability. Those with instability or deformity tended to have higher pain scores and Nurick grade, but a statistical correlation with outcome could not be determined from these small numbers.
Recurrence of myelopathy may occur with closure of the laminar door. We have switched to using rib allograft after closure of a laminar door in one patient. Half of our patients were treated with this method and this complication has not occurred. A variety of methods to stabilize the open-door have been described, and we recommend supplementing the standard open-door technique with additional modifications.24,32,36,39,45
Currently the senior author (CRC) secures the rib allograft with a suture which is threaded through a hole in the lateral mass, the medullary canal of the rib graft, and through a hole in the lamina and then tied. Additionally, laminoplasty does have the disadvantage of making decompression along the facet joint along the hinged side more difficult. This may be a consideration in patients with bilateral radiculopathy, although a keyhole foraminotomy may be performed to decompress a particular nerve root on the hinged side. We have not performed this procedure and believe that decompression alone is usually adequate.
Of the many reports of laminoplasty outcome, most have demonstrated substantial neurological recovery.3,13–15,18–20,29–31,35,42,47 These studies have included or have been composed entirely of patients with ossification of the posterior longitudinal ligament. Some studies have indicated a different natural history and operative outcome in patients with ossification of the posterior longitudinal ligament,26,36 and thus these patients were excluded. Additionally, the Japanese Orthopaedic Association scoring system for myelopathy, often present in the laminoplasty literature, is difficult to apply to Western patients. Herkowitz, using his own grading system, compared anterior cervical arthrodesis, laminectomy, and laminoplasty for multi-level spondylotic radiculopathy in 45 patients with a minimum 2 year follow-up and concluded that laminoplasty was an effective alternative to anterior cervical fusion and laminectomy.14 The complications of anterior arthrodesis and laminectomy could be avoided with laminoplasty. Patients with laminectomy had the poorest results.
Our series, too, demonstrated superior results with laminoplasty. We believe that laminoplasty is a safe and efficacious procedure in the management of selected patients with multiple level cervical spondylotic myelopathy or radiculopathy. Modified Nurick grades of myelopathy, pain scores, subjective and objective return of sensation and motor strength, and gait alterations demonstrated more substantial improvements in laminoplasty patients, with fewer late complications, than laminectomy. This must be balanced with the potential loss of cervical range-of-motion, neck stiffness, and the potential for transient neurologic injury encountered with laminoplasty. Additionally, several variables including preoperative Nurick grade, level of pain, postoperative Pavlov ratio, and space-available-for-the-cord can potentially indicate postoperative outcome and neurological recovery.
Footnotes
Investigation performed at the University of Iowa Hospitals and Clinics, Department of Orthopaedic Surgery, Iowa City, IA
References
- 1.Aita I, Hayashi K, Wadano Y, Yabuki Posterior Movement and Enlargement of the Spinal Cord After Cervical Laminoplasty. Spine. 1998;80B:33–37. doi: 10.1302/0301-620x.80b1.7919. [DOI] [PubMed] [Google Scholar]
- 2.Alvisi C, Borromei A, Cerisoli M, Giulioni M. Long-term Evaluation of Cervical Spine Disorders Following Laminectomy. J of Neurosurgical Sciences. 1988;32(3):109–112. [PubMed] [Google Scholar]
- 3.Baba H, Uchida K, Maezawa Y, Furusawa N, Wada M, Imura S. Three-dimensional Computed Tomography for Evaluation of Cervical Spinal Canal Enlargement After En-bloc Open-door Laminoplasty. Spinal Cord. 1997;35(10):674–679. doi: 10.1038/sj.sc.3100473. [DOI] [PubMed] [Google Scholar]
- 4.Callahan RA, Johnson RM, Margolis RN, Keggi KJ, Albright JA, Southwick WO. Cervical Facet Fusion for Control of Instability Following Laminectomy. J Bone Joint Surg. 1977;59A:991–1002. [PubMed] [Google Scholar]
- 5.Cattell HS, Clark GL. Cervical Kyphosis and Instability Following Multiple Laminectomies in Children. J Bone Joint Surg. 1967;49A:713–720. [PubMed] [Google Scholar]
- 6.Clark CR. Indications and Surgical Management of Cervical Myelopathy. Sem Spine Surg. 1989;1(4):254–261. [Google Scholar]
- 7.Clark E, Robinson PK. Cervical Myelopathy: A Complication of Cervical Spondylosis. Brain. 1956;79:483–487. doi: 10.1093/brain/79.3.483. [DOI] [PubMed] [Google Scholar]
- 8.Emery S, Bohlman HH, Bolesta M, Jones P. Anterior Cervical Decompression and Arthrodesis for the Treatment of Cervical Spondylotic Myelopathy: Two to Seventeen-Year Follow-up. J Bone and Joint Surg. 1998;80-A:941–951. doi: 10.2106/00004623-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Emery SE, Smith MD, Bohlman HH. Upper-airway Obstruction After Multiple Level Corpectomy for Myelopathy. J Bone Joint Surg. 1991;73-A:544–551. [PubMed] [Google Scholar]
- 10.Farey ID, McAfee PC, Davis RF, et al. Pseudoarthrosis of the Cervical Spine After Anterior Arthrodesis: Treatment by Posterior Nerve-root Decompression, Stabilization, and Arthrodesis. J Bone and Joint Surg. 1990;72-A:1171–1177. [PubMed] [Google Scholar]
- 11.Fernyhough JC, White JI, LaRocca H. Fusion Rates in Multilevel Cervical Spondylosis Comparing Allograft Fibula with Autograft Fibula in 126 Patients. Spine. 1991;16:561–564. doi: 10.1097/00007632-199110001-00022. [DOI] [PubMed] [Google Scholar]
- 12.Gregorius FK, Estrin T, Crandall PH. Cervical Spondylotic Radiculopathy and Myelopathy. A Long-term Follow-up Study. Arch of Neurology. 1976;33(9):618–625. doi: 10.1001/archneur.1976.00500090024005. [DOI] [PubMed] [Google Scholar]
- 13.Hase H, Watanabe T, Hirasawa Y, et al. Bilateral Open Laminoplasty Using Cervical Laminas for Cervical Myelopathy. Spine. 1991;16(11):1269–1276. doi: 10.1097/00007632-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Herkowitz HN. A Comparison of Anterior Cervical Fusion, Cervical Laminectomy, and Cervical Laminoplasty for the Surgical Management of Multiple Level Spondylotic Radiculopathy. Spine. 1988;13:774–780. doi: 10.1097/00007632-198807000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Herkowitz HN. Surgical Management of Cervical Disc Disease: "Open-Door" Laminoplasty. Seminars in Spine Surgery. 1989;1(4):245–253. [Google Scholar]
- 16.Herkowitz HN. The Surgical Management of Cervical Spondylotic Radiculopathy and Myelopathy. Clin Orthop. 1989;239:94–108. [PubMed] [Google Scholar]
- 17.Herman JM, Sonntag VKH. Cervical Corpectomy and Plate Fixation For Post-laminectomy Kyphosis. J Neurosurg. 1994;80:963–970. doi: 10.3171/jns.1994.80.6.0963. [DOI] [PubMed] [Google Scholar]
- 18.Hirabyashi K. Expansive Open-Door Laminoplasty for Cervical Spondylotic Myelopathy. Shujutu. 1978;32:1159–1163. [Google Scholar]
- 19.Hiarabayashi K, Bohlman HH. Multilevel Cervical Spondylosis. Laminoplasty Versus Anterior Decompression. Spine. 1995;20(15):1732–1734. doi: 10.1097/00007632-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Hirabyashi K, Satomi K. Operative Procedure and Results of Open-Door Laminoplasty. Spine. 1988;13:870–876. doi: 10.1097/00007632-198807000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Hosono N, Yonenobu K, Ono K. Neck and Shoulder Pain After Laminoplasty: a Noticeable Complication. Spine. 1996;21(7):1969–1973. doi: 10.1097/00007632-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hukudu S, Ogata M, Mochizuki T, Sichikawa K. Laminectomy Versus Laminoplasty for Cervical Myelopathy: Brief Report. J Bone Joint Surg. 1988;70B:325–326. doi: 10.1302/0301-620X.70B2.3346317. [DOI] [PubMed] [Google Scholar]
- 23.Inoue A, Ikata T, Katoh S. Spinal Deformity Following Surgery for Spinal Cord Tumors and Tumorous Lesions: Analysis Based on an Assessment of Spinal Functional Curve. Spinal Cord. 1996;34(9):53–62. doi: 10.1038/sc.1996.97. [DOI] [PubMed] [Google Scholar]
- 24.Itoh T, Tsuji H. Technical Improvements and Results of Laminoplasty for Compressive Myelopathy in the Cervical Spine. Spine. 1985;10:729–736. doi: 10.1097/00007632-198510000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Kamioka Y, Yamamoto H, Tani T, Ishida K, Sawamoto T. Postoperative Instability of Cervical OPLL and Cervical Radiculomyelopathy. Spine. 1989;14(11):1177–1183. doi: 10.1097/00007632-198911000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kawai, Sunago K, Doi K, Saik M, Taguchi T. Cervical Laminoplasty (Hattori's Method): Procedure and Follow-up Results. Spine. 1998;13:1245–1250. [PubMed] [Google Scholar]
- 27.Lonstein JE. Postlaminectomy Kyphosis. Clin Orthop. 1977;128:93–100. [PubMed] [Google Scholar]
- 28.Lonstein JE, Winter RB, Moe JH, Bradford DS, Chou SN, Pinto WC. Neurologic Deficits Secondary to Spinal Deformity: A Review of the Literature and Report of Forty-Three Cases. Spine. 1980;5:551–555. doi: 10.1097/00007632-198007000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kimura I, Oh-Hama M, Shingu H, Yonago K. Cervical Myelopathy Treated by Canal-Expansive Laminoplasty. J Bone Joint Surg. 1984;66-A:914–920. doi: 10.2106/00004623-198466060-00012. [DOI] [PubMed] [Google Scholar]
- 30.Kimura I, Shingu H, Nasu Y, et al. Long-term Follow-up of Cervical Spondylotic Myelopathy Treated By Canal-Expansive Laminoplasty. J Bone Joint Surg. 1995;77B:956–961. [PubMed] [Google Scholar]
- 31.Kohno K, Kumon Y, Oka Y, Matsui S, Ohue S, Sakaki S. Evaluation of Prognostic Factors Following Expansive laminoplasty for Cervical Spinal Stenotic Myelopathy. Surg Neurology. 1997;48(3):237–245. doi: 10.1016/s0090-3019(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee TT, Manzano GR, Green BA. Modified Open-door Cervical Expansive Laminoplasty For Cervical Spondylotic Myelopathy: Operative Technique, Outcome, and Predictors for Gait Improvement. J Neurosurg. 1997;86(1):64–68. doi: 10.3171/jns.1997.86.1.0064. [DOI] [PubMed] [Google Scholar]
- 33.Lees F, Aldren JW. Natural History and Prognosis of Cervical Spondylosis. Br Med J. 1972;95:1607–1610. doi: 10.1136/bmj.2.5373.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikawa Y, Shikara J, Yamamuro T. Spinal Deformity and Instability After Multilevel Cervical Laminectomy. Spine. 1987;12:6–11. doi: 10.1097/00007632-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Nagata K, Ohashi T, Abe J, Morita M, Inoue A. Cervical Myelopathy in Elderly Patients: Clinical Results and MRI Findings Before and After Decompression Surgery. Spinal Cord. 1996;34(4):220–226. doi: 10.1038/sc.1996.41. [DOI] [PubMed] [Google Scholar]
- 36.Nakano N, Nakano T, Nakano K. Comparison of the Results of Laminectomy and Open-Door Laminoplasty for Cervical Spondylotic Myeloradiculopathy and Ossification of the Posterior Longitudinal Ligament. Spine. 1988;13:792–794. doi: 10.1097/00007632-198807000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Nowinski GP, Vesarius H, Dipl-Ing, Nolte LP, et al. A Biomechanical Comparison of Cervical Laminaplasty and Cervical Facetectomy with Progressive Facetectomy. Spine. 1993;18:1995–2004. doi: 10.1097/00007632-199310001-00012. [DOI] [PubMed] [Google Scholar]
- 38.Nurick S. The Natural history and the Results of Surgical Treatment of the Spinal Cord Disorder Associated with Cervical Spondylosis. Brain. 1972;95:101–108. doi: 10.1093/brain/95.1.101. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien MF, Petersen D, Casey AT, Crockard H. A Novel Technique for Laminoplasty Augmentation of Spinal Canal Area Using Titanium Miniplate Stabilization: A Computerized Morphometric Analysis. Spine. 1996;21(4):474–483. doi: 10.1097/00007632-199602150-00012. [DOI] [PubMed] [Google Scholar]
- 40.Prolo DJ, Oklund SA, Butcher M. Toward Uniformity in Evaluating Results of Lumbar Spine Operations. A Paradigm Applied to Postoperative Lumbar Interbody Fusions. Spine. 1986;11(16):601–606. doi: 10.1097/00007632-198607000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Raynor RB, Pugh J, Shapiro I. Cervical Faccetectomy and its Effect on Spine Strength. J Neurosurg. 1985;63:278–282. doi: 10.3171/jns.1985.63.2.0278. [DOI] [PubMed] [Google Scholar]
- 42.Satomi K, Nishu Y, Kohno T, Hirabyashi K. Long-Term Follow-up Studies of Open-Door Expansive Laminoplasty for Cervical Stenotic Myelopathy. Spine. 1994;19:507–510. doi: 10.1097/00007632-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sim FH, Svien HJ, Bickel WH, Janes JM. Swan-neck Deformity Following Extensive Cervical Laminectomy. J Bone Joint Surg. 1974;56-A:564–580. [PubMed] [Google Scholar]
- 44.Symon L, Lavender P. The Surgical Treatment of Cervical Spondylotic Myelopathy. Neurology. 1967;17:117–127. doi: 10.1212/wnl.17.2.117. [DOI] [PubMed] [Google Scholar]
- 45.Tomita K, Nomura S, Umeda S, et al. Cervical Laminoplasty to Enlarge the Spinal Canal Multilevel Ossification of the Posterior Longitudinal Ligament With Myelopathy. Arch Orthop Trauma Surg. 1988;107:148–153. doi: 10.1007/BF00451594. [DOI] [PubMed] [Google Scholar]
- 46.Torg JS, Pavlov H, Genuario SE, et al. Neuropraxia of the Cervical Spinal Cord with Transient Quadriplegia. J Bone and Joint Surg. 1969;68-A:1354–1370. [PubMed] [Google Scholar]
- 47.Tsuji H. Laminoplasty for Patients With Compressive Myelopathy Due to So-called Spinal Canal Stenosis in Cervical and Thoracic Regions. Spine. 1982;7:28–34. doi: 10.1097/00007632-198200710-00002. [DOI] [PubMed] [Google Scholar]
- 48.White AA, Panjabi MM. Biomechanical Considerations in the Surgical Management of Cervical Spondylotic Myelopathy. Spine. 1988;7:856–860. doi: 10.1097/00007632-198807000-00029. [DOI] [PubMed] [Google Scholar]
- 49.Yonenobu K, Hosono N, Iwasaki M, et al. Laminoplasty Versus Subtotal Corpectomy. Spine. 1992;17:1281–1284. [PubMed] [Google Scholar]
- 50.Yonenobu K, Hosono N, Iwasaki M, Masatoshi A, Ono K. Neurologic Complications of Surgery for Compressive Myelopathy. Spine. 1991;16:1277–1282. doi: 10.1097/00007632-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Yonenobu K, Fuji T, Okada K, Fujiwara K, Yamashita K, Ono K. Causes of Neurologic Deterioration Following Surgical Treatment of Cervical Myelopathy. Spine. 1986;11:818–822. doi: 10.1097/00007632-198610000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida M, Otani K, Shibasaki K, Ueda S. Expansive Laminoplasty with Reattachment of Spinous Process and Extensor Musculature for Cervical Myelopathy. Spine. 1992;17:491–497. doi: 10.1097/00007632-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Zdelblick TA, Bohlman HH. Cervical Kyphosis and Myelopathy. J Bone and Joint. 1989;71-A:170–182. [PubMed] [Google Scholar]


